PrEP Provider Toolkit
Patient Eligibility
Careful Considerations
- Non-occupational postexposure prophylaxis (nPEP)
- Suspicion for HIV Acquisition During the Inter-test Reactivity Interval (ITRI)
- Delayed Seroconversion
- Other Considerations
Non-occupational postexposure prophylaxis (nPEP)
Postexposure prophylaxis (PEP) refers to the use of antiretroviral medications (ARVs) to prevent HIV acquisition when there was potential exposure in the past 72 hours.
There are two forms of PEP based on the setting where the exposure event occurred. It is termed occupational PEP (oPEP) if the exposure occurred among healthcare personnel in a healthcare setting and non-occupational PEP (nPEP) for all other scenarios.
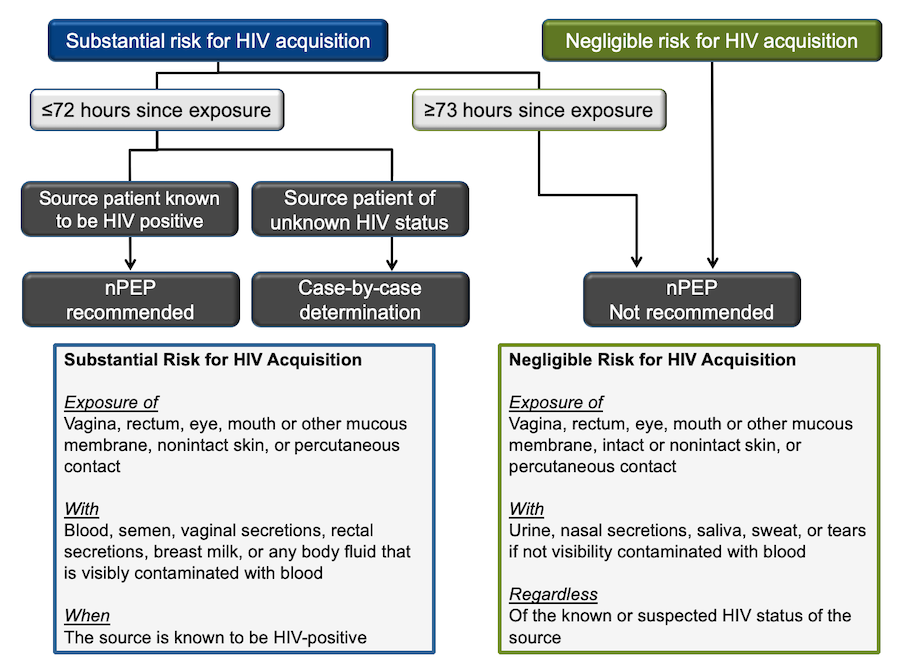
The following algorithm adopted from the National HIV Curriculum website was created using the 2016 CDC guidelines for nPEP (Figure 14). (19)
Centers for Disease Control and Prevention. (2018). Updated guidelines for antiretroviral postexposure prophylaxis after sexual, injection drug use, or other nonoccupational exposure to HIV—United States, 2016. U.S. Department of Health and Human Services.
https://www.cdc.gov/hiv/pdf/programresources/cdc-hiv-npep-guidelines.pdf
The first determination that needs to be made is the level of risk for HIV acquisition. Substantial risk is defined as direct exposure of mucosal sites or the bloodstream (non-intact skin or percutaneous) with specific bodily fluids (blood, genital fluids, or breastmilk) when the source patient is HIV+.
This is a good start though it requires you to know the HIV-status of the source patient, a detail typically unknown in cases that occur outside of medical settings. It also disregards the latest evidence of U=U. (15) The risk may be negligible if the event was sexual contact with an HIV+ source who has had undetectable viral levels for more than six consecutive months.
Eisinger, Dieffenbach, C. W., & Fauci, A. S. (2019). HIV viral load and transmissibility of HIV infection: undetectable equals untransmittable. JAMA: The Journal of the American Medical Association, 321(5), 451–452.
https://doi.org/10.1001/jama.2018.21167
Click image for full size view
Click the title of the figure to view source.
Figure 14 - Algorithm for use of non-occupational post-exposure prophylaxis (nPEP)
Source: Centers for Disease Control and Prevention, U.S. Department of Health and Human Services. Updated Guidelines for Antiretroviral Postexposure Prophylaxis After Sexual, Injection Drug Use, and Other Nonoccupational Exposure to HIV – United States, 2016.
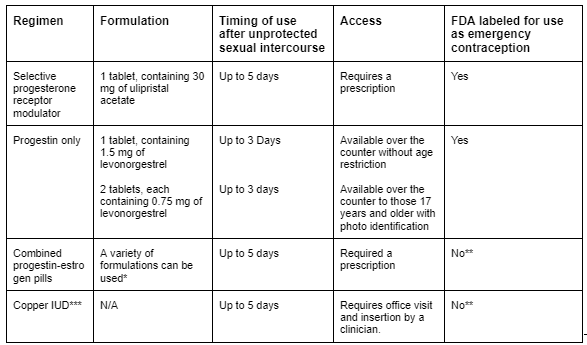
Table 3 - Available Methods of Emergency Contraception
Source: Emergency contraception. Practice Bulletin No. 152. American College of Obstetricians and Gynecologists. Obstet Gynecol 2015;126:e1–11.
In most cases, the substantial risk is hard to determine; thus, we must rely on our knowledge of HIV transmission probability (Figure 1) and other related factors (risk calculator).
Patients placed on nPEP should be asked if they would like to transition to PrEP after completion of the 28-day course. HIV-RNA and HIV-Ag/Ab should be collected during the last week of nPEP, and if they are both negative, patients can be switched to PrEP.
This can be a daunting and confusing “gray” area, which is why the National Clinician Consultation Center also has a dedicated hotline for nPEP questions.
(888) 448-4911 9am-8pm. Eastern Time Monday – Friday,
11am – 8pm. Eastern Time on weekends & holidays.
For further information, refer to the National HIV Curriculum website and the AETC’s nPEP toolkit.
One final note. The timing of nPEP roughly coincides with the timing of emergency contraception (EC). This critical component of care should be incorporated into the discussion when applicable. ACOG Guidelines on EC.
Suspicion for HIV Acquisition During the Inter-test Reactivity Interval (ITRI)
As previously discussed, the early stages of HIV infection pose many challenges for our laboratory and clinical approaches. In particular, the ITRI, which is the narrow time period when HIV-RNA is positive, but the HIV-Ag/Ab test is negative.
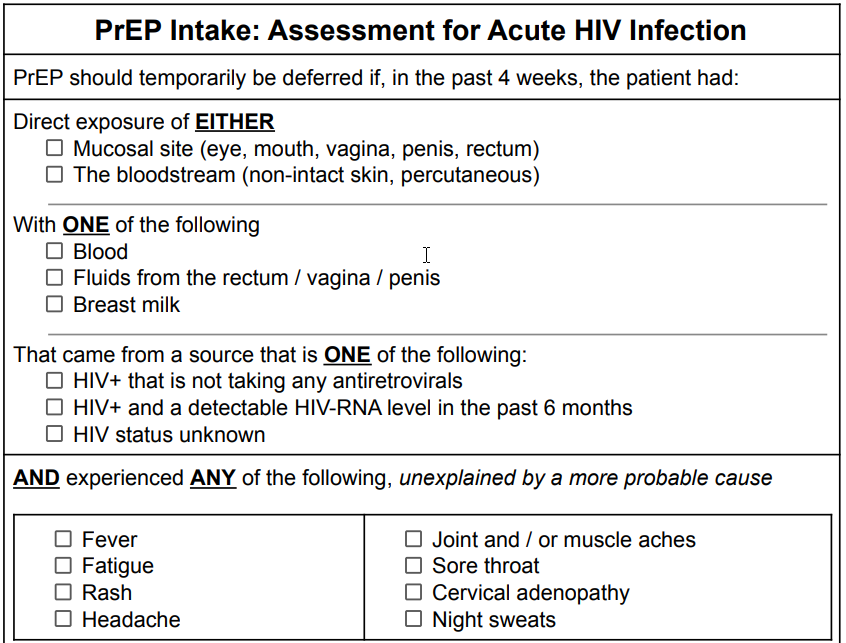
Current guidelines suggest...
PrEP should be deferred until HIV-RNA test results are obtained if, in the past four weeks, the patient had potential exposure to HIV AND experienced symptoms of the acute retroviral syndrome (ARS). (20)
Centers for Disease Control and Prevention. (2021). Preexposure prophylaxis for the prevention of HIV infection in the United States – 2021 update a clinical practice guideline. U.S. Department of Health and Human Services.
https://www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2021.pdf
Now, there are some underlying subtleties to this approach. First, as we now know, “potential exposures” can range widely in their risk of HIV transmission. For example, penile-vaginal sex with a condom poses a much lower risk than unprotected receptive anal intercourse.
For PrEP, there is no consensus as to where the threshold falls for what is considered a substantial risk. However, some parallels can be drawn to nPEP.
Second, given that ARS symptoms are inconsistent and obscure, we must consider the likelihood of other conditions. Perhaps, the patient with fever and fatigue was recently diagnosed with COVID-19. Delaying PrEP initiation would not be appropriate in this setting. Similar to the Well’s criteria for DVT and PE that asks if an alternative diagnosis is more likely, we suggest using other contextual factors to help make this decision.
A slight revision using these points of clarification would be…
PrEP should be deferred until HIV-RNA test results are obtained if, in the past four weeks, the patient had potential exposure to HIV with substantial risk AND experienced symptoms of the acute retroviral syndrome (ARS), unexplained by a more probable cause.
If the patient meets this suggested criteria, HIV-RNA and HIV-Ab/Ag should be collected and results reviewed prior to starting PrEP.
Again, utilize the National Clinicians Consultation Center hotline if you are ever in need of support.
(888) 448-4911 9am-8pm. Eastern Time Monday – Friday,
11am – 8pm. Eastern Time on weekends & holidays.
For further information, refer to the National HIV Curriculum website and the AETC’s nPEP toolkit.
Delayed Seroconversion
All of the medications in all three formulations of PrEP have activity against the HIV virus. They each require a certain level of concentration to ensure adequate protection. Since PrEP is a medication that relies heavily on patient adherence, there have been a few documented HIV infections that occurred during PrEP use. When drug levels fall below this critical threshold, HIV can permanently establish itself as a new infection. The residual amounts of PrEP medication that were inadequate in preventing infection go on to suppress the virus partially.
This partial suppression can sometimes delay the body’s natural immune response of creating antibodies. This phenomenon, called delayed seroconversion, can prolong the window period of HIV-Ag/Ab tests and thus, the ITRI.
The average time of delayed seroconversion for oral PrEP (F/TDF and F/TAF) is about 30 days (range 7-68 days) and for injectable PrEP (CAB), 80 days (range 35-185). (21) Because of this, there are minor changes for the lab recommendations when a patient was recently on PrEP.
Marzinke, Grinsztejn, B., Fogel, J. M., Piwowar-Manning, E., Li, M., Weng, L., McCauley, M., Cummings, V., Ahmed, S., Haines, C. D., Bushman, L. R., Petropoulos, C., Persaud, D., Adeyeye, A., Kofron, R., Rinehart, A., St Clair, M., Rooney, J. F., Pryluka, D., … Eshleman, S. H. (2021). Characterization of Human Immunodeficiency Virus (HIV) Infection in Cisgender Men and Transgender Women Who Have Sex With Men Receiving Injectable Cabotegravir for HIV Prevention: HPTN 083. The Journal of Infectious Diseases, 224(9), 1581–1592.
https://doi.org/10.1093/infdis/jiab152
Other Considerations
Although they are not strong contraindications, the following details warrant careful consideration before initiating PrEP. Please do not let the details of this section deter you from discussing and providing PrEP with your patients. These intricate aspects of PrEP are included to fine-tune your understanding and address some common questions that may arise.
| Pregnancy and breastfeeding |
There is an increased risk of acquiring HIV during periods of conception, pregnancy, and breastfeeding. (22). F/TDF (Truvada) is the recommended agent for this patient population; it does not cause any adverse pregnancy outcomes. The effects on infants exposed to F/TDF during lactation have not been well studied though existing studies suggest very limited drug exposure. (23) Mugwanya, John-Stewart, G., & Baeten, J. (2017). Safety of oral tenofovir disoproxil fumarate-based HIV pre-exposure prophylaxis use in lactating HIV-uninfected women. Expert Opinion on Drug Safety, 16(7), 867–871. Benaboud, Pruvost, A., Coffie, P. A., Ekouévi, D. K., Urien, S., Arrivé, E., Blanche, S., Théodoro, F., Avit, D., Dabis, F., Tréluyer, J.-M., & Hirt, D. (2011). Concentrations of tenofovir and emtricitabine in breast milk of HIV-1-infected women in Abidjan, Côte d’Ivoire, in the ANRS 12109 TEmAA study, step 2. Antimicrobial Agents and Chemotherapy, 55(3), 1315–1317. Waitt, Olagunju, A., Nakalema, S., Kyohaire, I., Owen, A., Lamorde, M., & Khoo, S. (2018). Plasma and breast milk pharmacokinetics of emtricitabine, tenofovir and lamivudine using dried blood and breast milk spots in nursing African mother-infant pairs. Journal of Antimicrobial Chemotherapy, 73(4), 1013–1019. Mugo, Heffron, R., Donnell, D., Wald, A., Were, E. O., Rees, H., Celum, C., Kiarie, J. N., Cohen, C. R., Kayintekore, K., & Baeten, J. M. (2011). Increased risk of HIV-1 transmission in pregnancy: a prospective study among African HIV-1-serodiscordant couples. AIDS (London), 25(15), 1887–1895. Thomson, Hughes, J., Baeten, J. M., John-Stewart, G., Celum, C., Cohen, C. R., Ngure, K., Kiarie, J., Mugo, N., & Heffron, R. (2018). Increased risk of HIV acquisition among women throughout pregnancy and during the postpartum period: a prospective per-coital-act analysis among women with HIV-infected partners. The Journal of Infectious Diseases, 218(1), 16–25 Further investigation is needed for F/TAF (Descovy) and CAB (Apretude) in this patient population. Existing data among HIV+ women taking these agents for HIV treatment does show favorable safety profiles. Use of these agents as PrEP may be considered if there is a shared and informed decision that the risks outweigh the benefits. Information should be anonymously submitted if any form of PrEP is used during pregnancy at http://www.apregistry.com/ |
| Disorders that affect bone mineral density (BMD) |
Both F/TDF (Truvada) and F/TAF are known to lower BMD. Although F/TDF (Truvada) is often cited as the more “toxic” agent on bone health when compared to F/TAF (Descovy), all existing studies have found only small and clinically insignificant impacts on BMD. Furthermore, the minor losses in BMD caused by F/TDF (Truvada) were fully recovered within 12-18 months of drug discontinuation. (24) Jones, Restrepo, D., Kasowitz, A., Korenstein, D., Wallenstein, S., Schneider, A., & Keller, M. J. (2007). Risk factors for decreased bone density and effects of HIV on bone in the elderly. Osteoporosis International, 19(7), 913–918. Cazanave, Dupon, M., Lavignolle-Aurillac, V., Barthe, N., Lawson-Ayayi, S., Mehsen, N., Mercié, P., Morlat, P., Thiébaut, R., & Dabis, F. (2008). Reduced bone mineral density in HIV-infected patients: prevalence and associated factors. AIDS (London), 22(3), 395–402. Brown, & Qaqish, R. B. (2006). Antiretroviral therapy and the prevalence of osteopenia and osteoporosis: a meta-analytic review. AIDS (London), 20(17), 2165–2174. Nevertheless, preference towards F/TAF (Descovy) or CAB (Apretude) may be warranted in those with prior pathologic fracture and/or pre-existing conditions related to poor bone health such as: osteopenia/osteoporosis, type I diabetes, celiac disease (or other malabsorptive conditions), hyperthyroidism, hyperparathyroidism, osteogenesis imperfecta, multiple myeloma, chronic use of glucocorticosteroids or hormone blockers. None of these conditions are hard contraindications to PrEP use and it should be discussed that BMD declines are much more significant and irreversible once HIV is contracted. |
| Drugs with potentially concerning interactions |
Aside from the previously mentioned drugs that are absolutely contraindicated, other drugs may cause interactions that warrant careful monitoring for toxicities. Especially those that are nephrotoxic. Utilize https://hiv-druginteractions.org/checker to make informed decisions for your patients. |
| Hepatitis B virus (HBV) infection |
The medications in oral formulations of PrEP (F/TDF and F/TAF) also have activity against HBV. Among patients with a positive hepatitis B surface antigen (HBsAg), abrupt discontinuation of oral PrEP can potentially cause hepatic flares. More recent analyses have gone on to say that HBsAg+ individuals with normal or near normal transaminase levels have an extremely low risk of hepatic flare when stopping PrEP. The CDC guidelines specifically states that PrEP should not be withheld while waiting for HBV results. (25) Nevertheless, the potential of causing hepatic flares is not to be taken lightly and due to this concept, ED-PrEP is not recommended for patients who are HBsAg+. |
| Patients ≥ 65 years old |
No direct studies have been performed on this age group. Kidney function and bone mineral density naturally deteriorates with age so caution should be used with select PrEP agents. |
| Table 5. Careful considerations regarding PrEP Use | |