PrEP Provider Toolkit
| Site: | Pacific AIDS Education & Training Center |
| Course: | PrEP Provider Toolkit |
| Book: | PrEP Provider Toolkit |
| Printed by: | Guest user |
| Date: | Thursday, November 21, 2024, 2:48 AM |
WELCOME
Welcome
Thank you for your interest in the Pacific AIDS Education and Training Center PrEP toolkit for providers.
Welcome to the Pacific AIDS Education and Training Center’s provider toolkit for pre-exposure prophylaxis (PrEP). You are about to begin an educational session that will significantly improve the quality of care you provide and help contribute to the global efforts to eradicate the Human Immunodeficiency Virus (HIV).
Since its discovery in the 1980s, HIV has been a major public health issue that continues to affect millions worldwide. Fortunately, incredible medical advancements have been made in this field that not only significantly improve the lives of those living with HIV, but can also effectively prevent the occurrence of new infections.
PrEP is one of modern medicine’s most powerful tools in disease prevention as it is highly efficacious, well-tolerated, and cost-effective. However, despite its existence for the past decade (since 2012), utilization has been fairly limited due to provider knowledge, comfort, and deliverability barriers.
This site is specifically designed to improve your understanding and confidence in providing this essential tool. You will be guided through the most relevant and practical aspects of PrEP care and be provided with various resources to help you streamline PrEP into your everyday practice.
This toolkit primarily utilizes information from the Center for Disease Control and Prevention’s (CDC) PrEP Clinical Guidelines, updated in December 2021. Minor modifications of these guidelines will be suggested to reduce any confusion that may deter providers from utilizing PrEP while allowing for more comprehensive care that doesn’t jeopardize safety.
The information presented here will be directed towards clinicians and, at times, may get a bit technical. But rest assured that if you need further support and guidance, there is a free hotline available to help you address any and all aspects of PrEP care.
National Clinicians Consultation Center
855-HIV-PrEP (855-448-7737) Monday – Friday, 9am – 8pm Eastern Time
Voice mails can be left at any time.
Lastly, PrEP delivery and management are not meant to fall solely onto the shoulders of providers. Like all the other conditions you manage, the prevention of HIV with PrEP is best performed with a team approach, utilizing everyone in your practice setting from the front desk to the back office. However, if you decide that PrEP is not something you want to provide, patients can be directed to resources to learn more about PrEP and find a PrEP provider.
← Browse the tabs to learn more.
Use the navigation in the lower right corner ↘ to begin the training.
What is the Pacific AETC?
 The
Pacific AIDS Education and Training Center (Pacific AETC) is a member of a national
AIDS Education and Training Center network of eight regional and three national
centers, covering all 50 states, as well as US Territories and Jurisdictions.
Funding is provided by the Health Resources and Services Administration (HRSA)
HIV/AIDS Bureau through the Ryan White Program, Part F.
The
Pacific AIDS Education and Training Center (Pacific AETC) is a member of a national
AIDS Education and Training Center network of eight regional and three national
centers, covering all 50 states, as well as US Territories and Jurisdictions.
Funding is provided by the Health Resources and Services Administration (HRSA)
HIV/AIDS Bureau through the Ryan White Program, Part F.
Pacific AETC works to expand the number and ability of healthcare professionals and organizations in the Pacific region to provide high-quality HIV-related services to increase access to healthcare and decrease health inequities. We work with health care providers, clinics, health jurisdictions, and policymakers in Arizona, California, Hawai`i, Nevada, and the US Affiliated Pacific Island Jurisdictions.
A network of eight Local Partners (LPs) provides services on local and regional levels. Five LPs are located throughout California, with state-wide programs in Arizona, Nevada, and Hawai’i. The Hawai’i LP also covers the six U.S. Pacific Jurisdictions (Guam, American Samoa, Federated States of Micronesia, Marshall Islands, North Mariana Islands, and Palau).
Each LP has its roster of qualified faculty, programs, and activities designed to meet their local region’s or state’s needs. LPs are based at academic medical centers, with access to highly qualified HIV specialists and community expertise. Contact your local AETC for details about training and capacity-building opportunities. Continuing education credit is available for many activities.
Target Audience
![]() Pacific AETC offers
education, training, and capacity-building programs specifically designed to
improve HIV/AIDS care, treatment, and prevention services for ALL interdisciplinary
team members. This includes but is not limited to:
Pacific AETC offers
education, training, and capacity-building programs specifically designed to
improve HIV/AIDS care, treatment, and prevention services for ALL interdisciplinary
team members. This includes but is not limited to:
- Mental Health Providers
- Social Workers
- Case Managers
- Physicians
- Nurses
- Advance Practice Nurses
- Physician Assistants
- Dentists
- Pharmacists
- Community Health Workers
Local Partners & State Office of AIDS
A network of eight Local Partners (LPs) provides services on local and regional levels. Five LPs are located throughout California, with one each in Arizona, Nevada, and Hawai’i. The Hawai’i LP also covers the six U.S. Pacific Jurisdictions (Guam, American Samoa, Federated States of Micronesia, Marshall Islands, North Mariana Islands, and Palau).
Each LP has its roster of qualified faculty, programs, and activities designed to meet their local region’s or state’s needs. LPs are based at academic medical centers, with access to highly qualified HIV specialists. Contact your regional AETC for details about training and capacity building opportunities. Continuing education credit is available for many activities.
Basics of HIV
 Understanding the disease in which we
are trying to prevent is important to truly value the power and potential of PrEP. In this section, we will be covering how HIV is transmitted, how we test for HIV, how new HIV infections present, and important public health statistics about HIV.
Laying down this foundational understanding will help make sense of the information provided in the following sections.
Understanding the disease in which we
are trying to prevent is important to truly value the power and potential of PrEP. In this section, we will be covering how HIV is transmitted, how we test for HIV, how new HIV infections present, and important public health statistics about HIV.
Laying down this foundational understanding will help make sense of the information provided in the following sections.
Transmission risk factors
To truly understand and appreciate the potential of PrEP, it is worthwhile to briefly review the disease it prevents. HIV is a retroviral infection that specifically attacks the CD4 cells in the immune system.
Transmission of HIV typically occurs through contact with blood or fluids from the rectum, penis, or vagina. Additionally, HIV can be transmitted from a mother to her child perinatally or through breastfeeding. Exposure of HIV to damaged mucosal tissue or directly into the bloodstream is required for transmission. Exposure to HIV on intact skin does not pose a risk, nor do acts such as hugging, kissing, or sharing utensils.
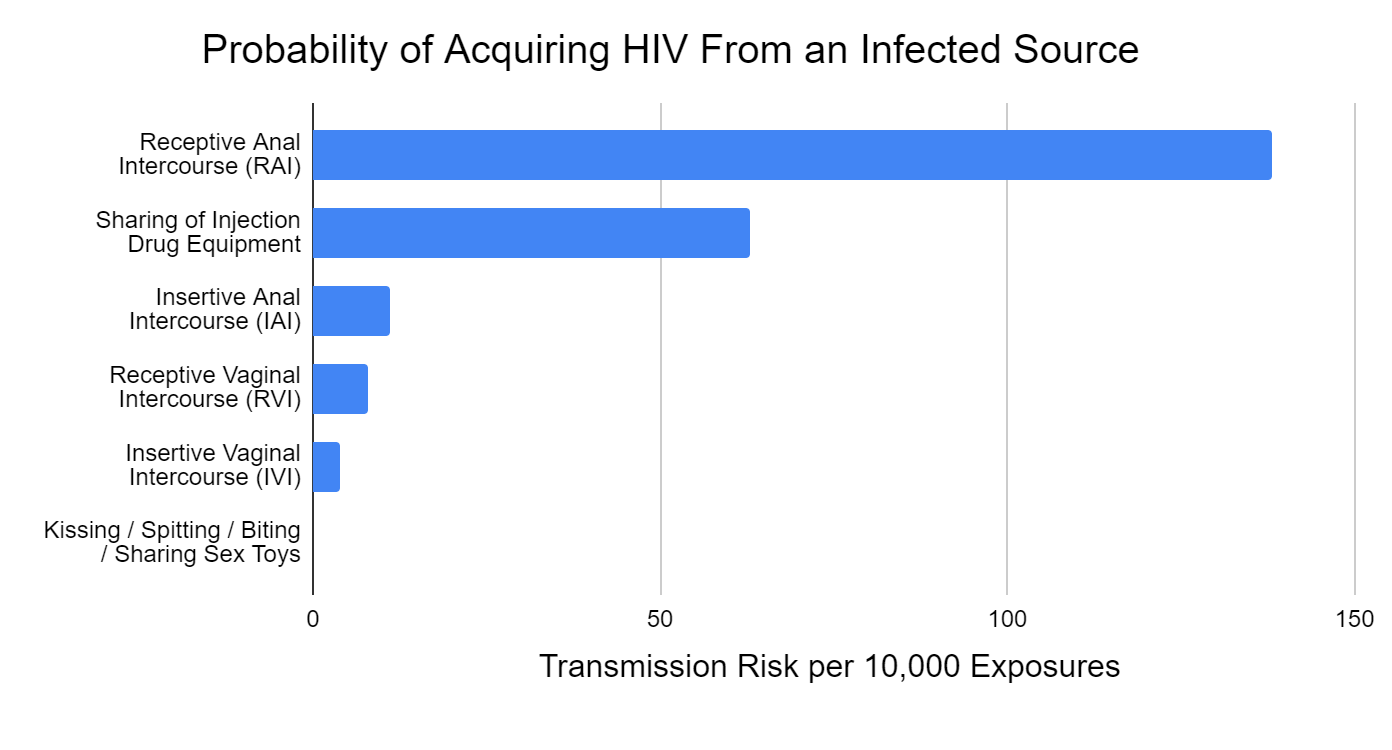
Figure 1 depicts the estimated likelihood of acquiring HIV from an infected source based on the type of exposure. Note that the second highest risk factor occurs among people who inject drugs (PWIDs), which is a population of rising concern. For further information about HIV transmission: https://www.cdc.gov/hiv/basics/transmission.html
Click for image for full size view
Click the title of the figure to view source.
Figure 1. - Estimated Probability of Acquiring HIV from an Infected Source by Exposure Type
Source: Patel, Borkowf, C. B., Brooks, J. T., Lasry, A., Lansky, A., & Mermin, J. (2014). Estimating per-act HIV transmission risk: a systematic review. AIDS (London), 28(10), 1509–1519. https://doi.org/10.1097/QAD.0000000000000298
Pretty, Anderson, G. S., & Sweet, D. J. (1999). Human bites and the risk of human immunodeficiency virus transmission. The American Journal of Forensic Medicine and Pathology, 20(3), 232–239. https://doi.org/10.1097/00000433-199909000-00003
Factors contributing to HIV transmission are fundamentally important to understand as it helps discern which patients are at the highest risk and how to protect them best.
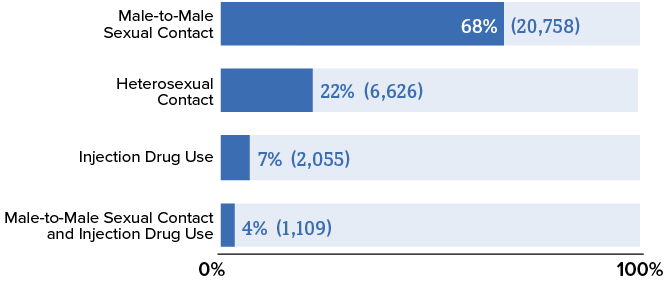
Anal sex or sex under the influence of drugs and alcohol has a higher likelihood of damaging the protective mucosal layer, which facilitates HIV transmission. Local inflammation caused by sexually transmitted infections (STI) also decreases the integrity of the mucosal lining. This partly explains why groups such as men who have sex with men (MSM) have higher rates of HIV transmission (Figure 2).
The CDC has a helpful tool that estimates the risk of sexually transmitted HIV based on various factors. It provides a powerful graphic that can be used with patients during visits to improve their self-perceived risk: https://hivrisk.cdc.gov/risk-estimator-tool/#-mb|iai
Figure 2. - New HIV Diagnoses in the US by Transmission Category, 2020
Source: Centers for Disease Control and Prevention. (2022). Diagnoses of HIV infection in the United States and dependent areas, 2020. U.S. Department of Health and Human Services. https://www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance-report-2020-updated-vol-33.pdf
HIV Testing
The recommended tests for HIV can vary based on the clinical scenario and the type of PrEP medication being utilized. Let’s briefly review this to build a foundational understanding of HIV testing options, their indications, and their limitations.
HIV-RNA
- Sometimes called nucleic acid test (NAT) or “viral load”
- Measures the amount of circulating HIV genetic material
- Becomes detectable ~10 days after HIV acquisition
p24 antigen (Ag)
- A structural protein found in the HIV capsid
- The antigen component of combined HIV-Ag/Ab testing.
- Becomes detectable ~18 days after HIV acquisition
HIV antibody (Ab)
- Antibodies against HIV; starts with IgM which gets replaced over time with IgG
- The antibody component of combined HIV-Ag/Ab testing
- Becomes detectable ~24 days after HIV acquisition
Click image for full size view
Click the title of the figure to view source.
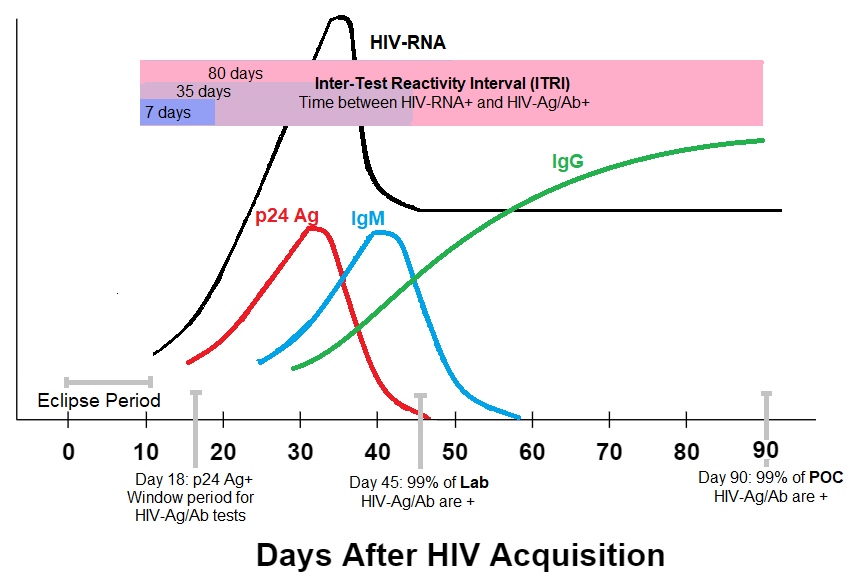
Figure 3. - Timing of HIV Test Positivity After Acquisition
Fiebig, Wright, D. J., Rawal, B. D., Garrett, P. E., Schumacher, R. T., Peddada, L., Heldebrant, C., Smith, R., Conrad, A., Kleinman, S. H., & Busch, M. P. (2003). Dynamics of HIV viremia and antibody seroconversion in plasma donors: implications for diagnosis and staging of primary HIV infection. AIDS (London), 17(13), 1871–1879.
https://doi.org/10.1097/00002030-200309050-00005
Branson, B.M., Owen, M.S., Wesolowshi, L.G., Bennett, B., Werner, B.G., Wroblewski, K.E., Pentella, M.A. (2014). Laboratory testing for the diagnosis of HIV infection: updated recommendations.
https://doi.org/10.15620/cdc.23447
Delaney, Hanson, D. L., Masciotra, S., Ethridge, S. F., Wesolowski, L., & Owen, S. M. (2017). Time until emergence of HIV test reactivity following infection with HIV-1: implications for interpreting test results and retesting after exposure. Clinical Infectious Diseases, 64(1), 53–59.
https://doi.org/10.1093/cid/ciw666
Newly acquired HIV infections take some time to make substantial copies of its genome. During this eclipse period, which typically lasts 10-12 days, we are unable to detect an HIV infection with any type of test. This eclipse period also happens to be the window period for HIV-RNA tests.
The window period is the time between HIV acquisition and lab test positivity.
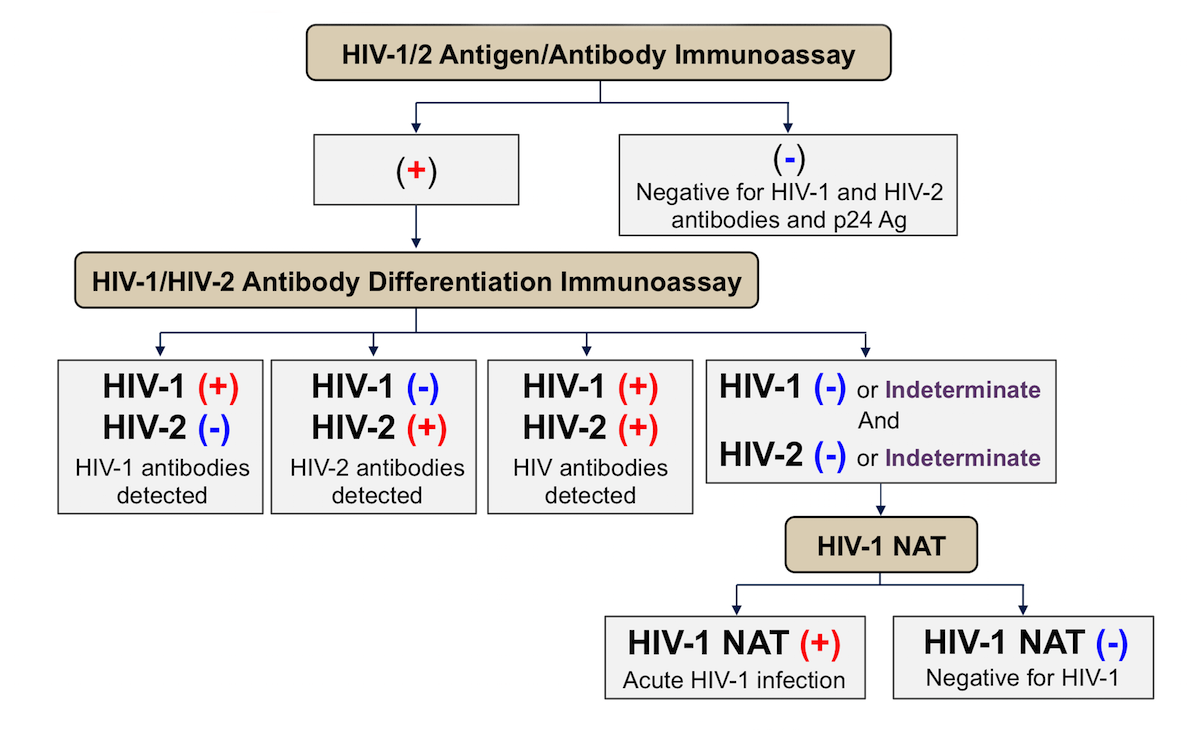
Each successive generation of HIV-Ag/Ab testing has worked to shorten the window period to allow for earlier diagnosis. The currently recommended and most commonly used HIV-Ag/Ab 4th-generation test checks for the p24 Ag, HIV-IgM, and HIV-IgG. The testing algorithm is presented in Figure 4. This test can be performed in the laboratory or as a point-of-care (POC). The only POC HIV-Ag/Ab test recommended in the context of PrEP is the Abbott Determine HIV-1/2 Ag/Ab Combo, a fingerstick test that generates results in 20 minutes. (1)
DetermineTM HIV-1/2 Ag/Ab Combo. (n.d.). Retrieved September 19, 2022, from
https://www.globalpointofcare.abbott/en/product-details/determine-1-2-ag-ab-combo.html
Figure 4 - HIV-Ag/Ab testing algorithm
Source: Centers for Disease Control and Prevention. (2018). Quick reference guide: recommended laboratory HIV testing algorithm for serum or plasma specimens. U.S. Department of Health and Human Services.
https://www.cdc.gov/hiv/pdf/guidelines_testing_recommendedlabtestingalgorithm.pdf
The window period for a laboratory based 4th-generation HIV-Ag/Ab test is between 18-45 days; the point-of-care (POC) version has a slightly longer window period of 18-90 days. After the window period, these tests will detect 99% of all newly acquired HIV infections.
Although HIV-RNA tests have the shortest window period, we do not routinely rely on this test for screening as it is costly and places a high burden on laboratories.
HIV-RNA is typically only used in the following scenarios:
- Monitoring disease activity in someone with a known HIV infection
- Checking for HIV acquisition during the inter-test reactivity interval
The inter-test reactivity interval (ITRI) is the time between HIV-RNA test positivity and HIV-Ag/Ab test positivity.
As highlighted in purple boxes in Figure 3, this ITRI can be as short as a week to as long as 80 days. Thankfully, it has been determined that the ITRI of current Ag/Ab tests to capture 95% of new infections is just one week. (2) In other words, the current HIV-Ag/Ab tests that we use can reliably detect new infections just a week after HIV-RNA tests can.
Delaney, Hanson, D. L., Masciotra, S., Ethridge, S. F., Wesolowski, L., & Owen, S. M. (2017). Time until emergence of HIV test reactivity following infection with HIV-1: implications for interpreting test results and retesting after exposure. Clinical Infectious Diseases, 64(1), 53–59.
https://doi.org/10.1093/cid/ciw666
However, this indicates that clinical scenarios still exist where HIV-RNA testing is needed before starting PrEP. We will be covering this in detail in the coming sections. Admittedly, this is the most challenging portion of PrEP care though it does not need to be applied in the vast majority of cases, so do not be deterred.
The recommended tests for HIV can vary based on the clinical scenario and the type of PrEP medication being utilized. Let’s briefly review this to build a foundational understanding of HIV testing options, their indications, and their limitations.
Acute HIV infections
The early stages of an HIV infection present many challenges in clinical decision making. In the previous section, we covered how objective measures can vary based on the timing of infection. Using subjective measures in the clinical setting is even more challenging, if not impossible, to accurately diagnose acute retroviral syndrome (ARS).
Only half of new HIV infections are estimated to cause symptoms between 2-4 weeks after acquisition. Among those who reported symptoms, only fever and fatigue occurred in most cases. Other less commonly reported symptoms include myalgia, rash, headache, sore throat, cervical adenopathy, joint aches, night sweats, and diarrhea. (3) This leads to a very broad differential diagnosis. Taken altogether, symptoms of ARS are both unreliable and extremely nonspecific; and thus, should not be used in isolation to make clinical decisions.
Daar, Pilcher, C. D., & Hecht, F. M. (2008). Clinical presentation and diagnosis of primary HIV-1 infection. Current Opinion in HIV & AIDS, 3(1), 10–15.
https://doi.org/10.1097/COH.0b013e3282f2e295
Furthermore, after this short period of time when symptoms occur, patients can go on for years without any new complaints. In fact, despite collective efforts to perform more routine testing, it is estimated that about 13% of people living with HIV/AIDS (PLWHA) are unaware of their diagnosis and account for over a third of new infections. (4)
Centers for Disease Control and Prevention. (2021). Estimated HIV incidence and prevalence in the United States, 2015–2019. U.S. Department of Health and Human Services.
https://www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance-supplemental-report-vol-26-1.pdf
How, then, can we improve our clinical gestalt for a condition that presents so ambiguously and has such an enormous impact on the lives of individuals and communities?
The answer to this question is complex, but it relies on several key points.
- An environment and approach that fosters patient comfort and trust is necessary to start relevant conversations
- Thorough and accurate sexual and substance use histories must be obtained
- A firm understanding of HIV tests must be developed and they should be utilized judiciously.
We will cover techniques and frameworks to help you improve these skills in the coming sections.
Important HIV Epidemiology
Prevalence captures the proportion of a population that currently lives with a condition. Incidence captures the proportion of a population that is newly infected with a condition.
Medical advancements such as antiretroviral therapy (ART) contribute to longer life expectancies among people living with HIV/AIDS (PLWHA) and, thus, rising prevalence rates. When it comes to PrEP, we are more concerned with incidence rates as it is a preventive tool to avoid new infections from occurring.
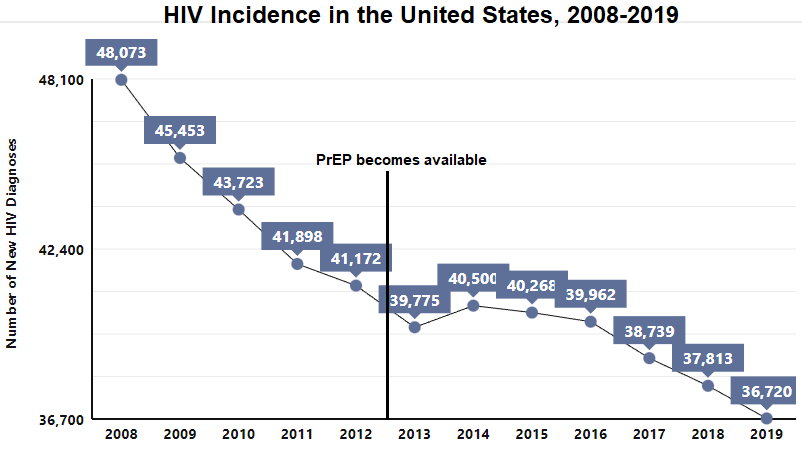
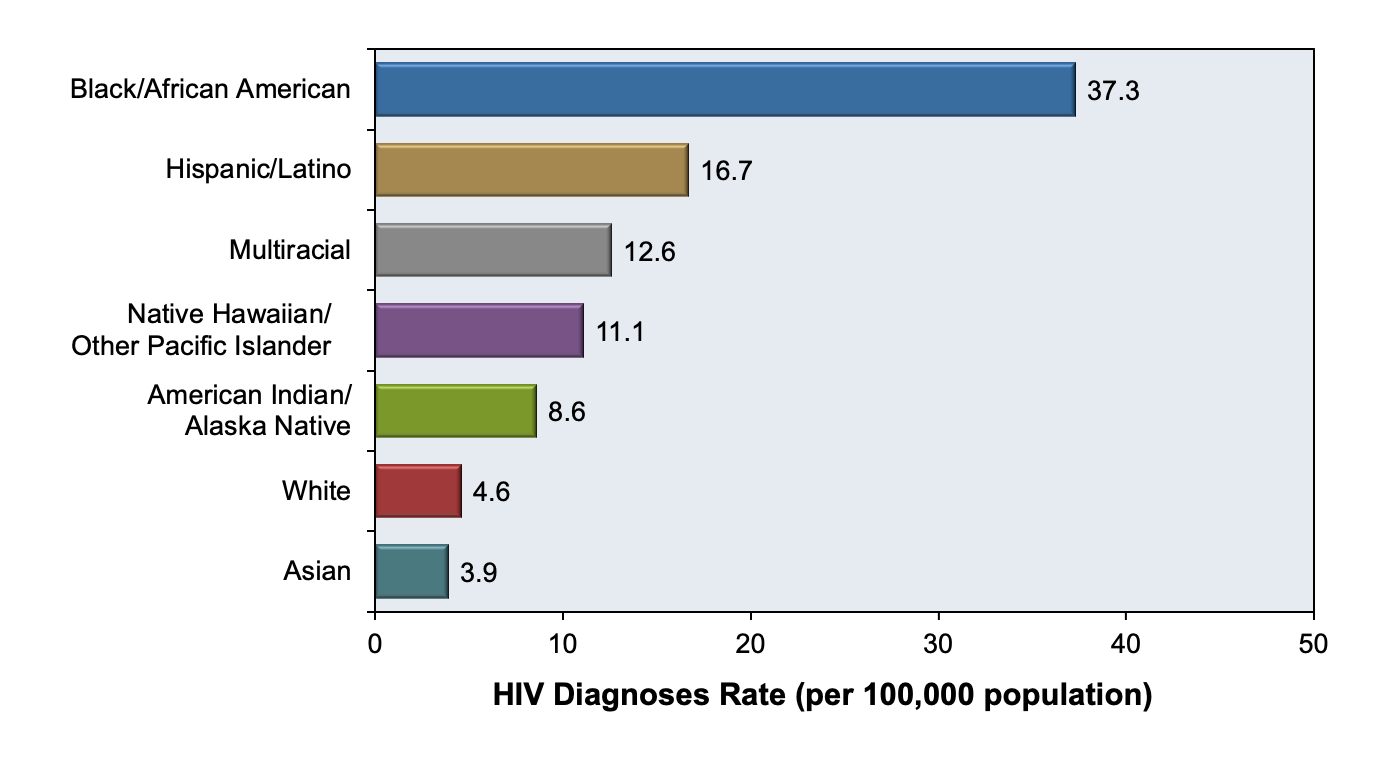
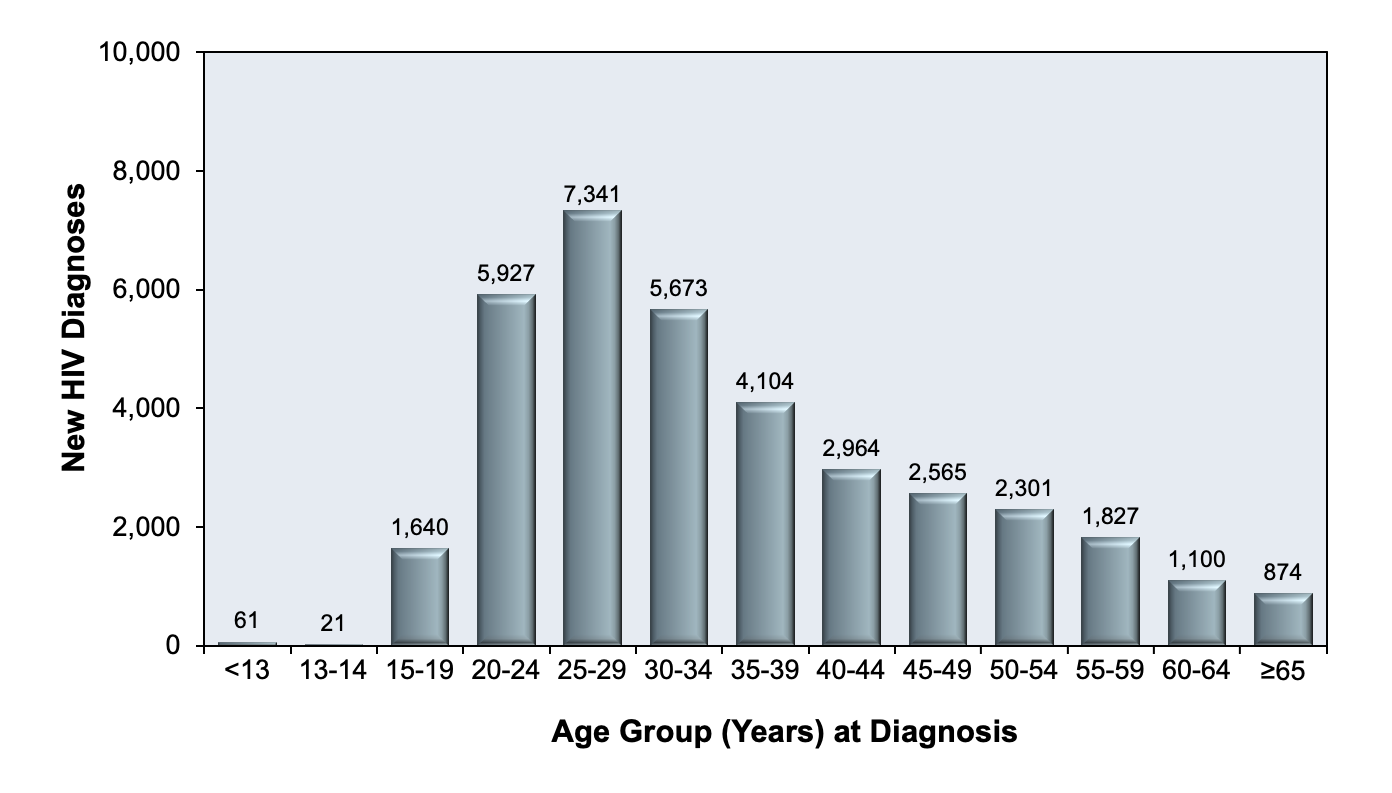
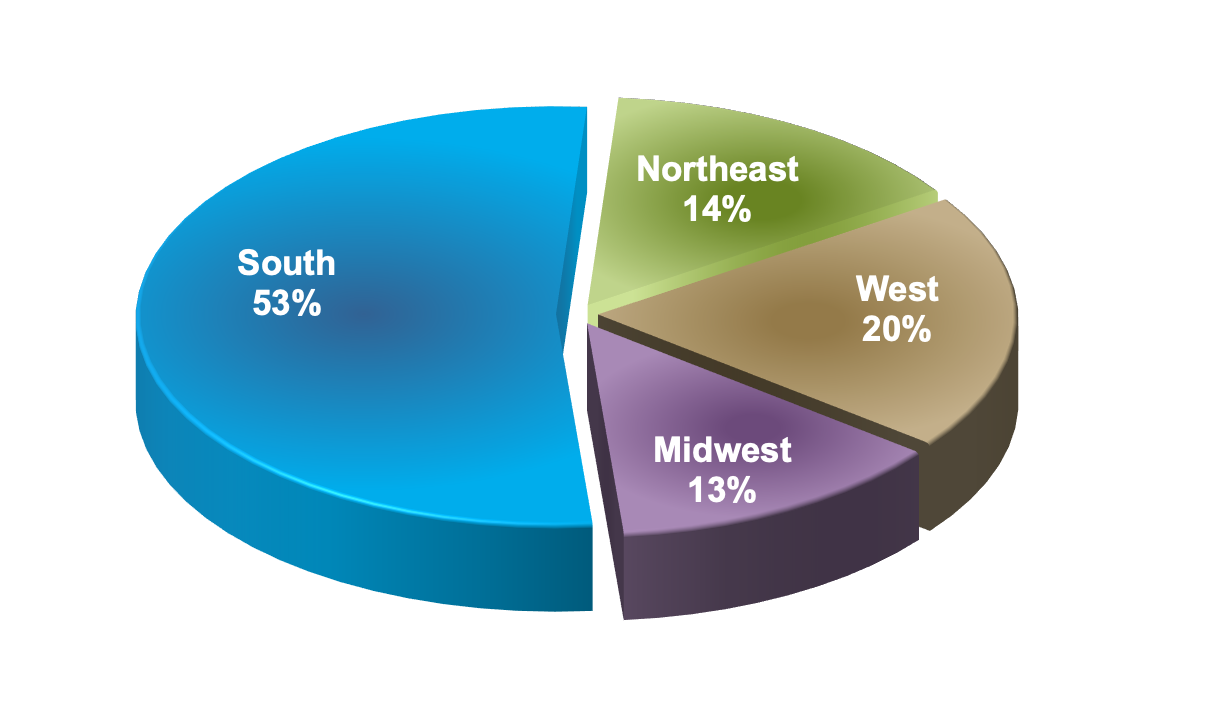
Figure 5 shows that the incidence of HIV in the United States has been steadily declining over the years. Despite this seemingly reassuring trend, when viewed with a closer lens, concerning disparities are revealed across racial groups, age groups, and geographic regions. HIV disproportionately affects Black/African Americans between the ages of 20-34, and a startling majority of all new HIV cases occur in Southern states alone (Figures 6-8). (5)
Centers for Disease Control and Prevention. (2021). Diagnoses of HIV infection in the United States and dependent areas, 2019. U.S. Department of Health and Human Services.
https://www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance-report-2018-updated-vol-32.pdf
It is also worth pointing out that the major reductions in HIV incidence occurred before PrEP was introduced. Improved testing and treatment of those living with HIV account for these early changes in incidence rates. This highlights the rather limited impact PrEP has had on this crucial statistic.
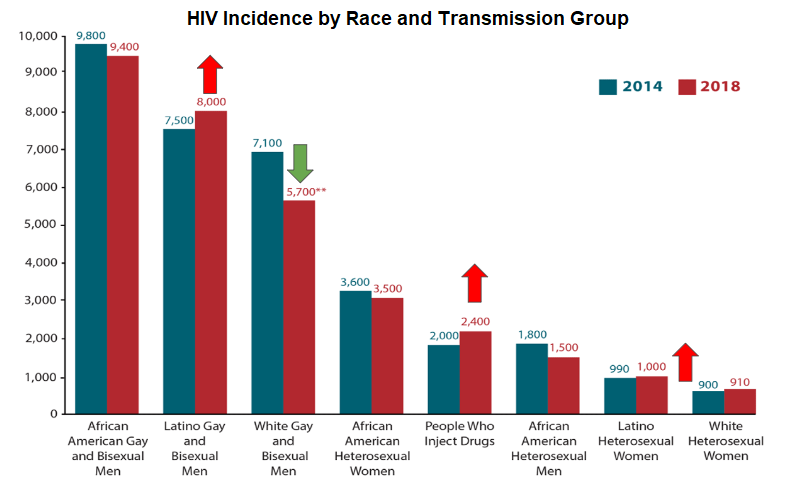
Since its availability, PrEP has predominantly benefited white gay and bisexual men. HIV incidence is worsening among groups of growing concern, such as Latino men and women and people who inject drugs (PWIDs) (Figure 9).
Most of the graphics shown below have been adopted from the National HIV Curriculum, a free resource created by the University of Washington and funded by the Health Resources and Services Administration (HRSA). It includes easy to follow, self-guided modules that provide an in-depth understanding of HIV: https://www.hiv.uw.edu/
They also make resources to improve your understanding of STIs, Hepatitis B, and Hepatitis C. Both CME and CNE credits can be earned by completing these self-study modules.
Click image for full size view
Click the title of the figure to view source.
Figure 5 - Incidence Rates in the United States, 2008-2019
Figure 6 - Incidence Rates in the United States in 2019, by Race/Ethnicit
Source: Centers for Disease Control and Prevention. (2021). Diagnoses of HIV infection in the United States and dependent areas, 2019. U.S. Department of Health and Human Services.
https://www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance-report-2018-updated-vol-32.pdf
Graph obtained for the HIV National Curriculum at www.hiv.uw.edu. Accessed September 1, 2022.
Figure 7 - HIV Incidence in the United States in 2019, by Age Group
Source: Centers for Disease Control and Prevention. (2021). Diagnoses of HIV infection in the United States and dependent areas, 2019. U.S. Department of Health and Human Services.
https://www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance-report-2018-updated-vol-32.pdf
Graph obtained for the HIV National Curriculum at www.hiv.uw.edu. Accessed September 1, 2022
Figure 8 - New HIV Diagnosis in the United States in 2019, by Region of Residence
Source: Centers for Disease Control and Prevention. (2021). Diagnoses of HIV infection in the United States and dependent areas, 2019. U.S. Department of Health and Human Services.
https://www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance-report-2018-updated-vol-32.pdf
Graph obtained for the HIV National Curriculum at www.hiv.uw.edu. Accessed September 1, 2022.
Figure 9 - New HIV Diagnosis in the United States in by Race and Transmission Group, 2014-2018
Source: Centers for Disease Control and Prevention. (2021). Diagnoses of HIV infection in the United States and dependent areas, 2019. U.S. Department of Health and Human Services. https://www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance-report-2018-updated-vol-32.pdf
Patient-centered approach
 Discussing PrEP naturally requires very sensitive information to be shared. A mindful and patient-centered approach is necessary to instill the comfort and build proper rapport. In this section, we will review gender-related terminology and how to effectively obtain a sexual and substance use history. Tailoring your approach will continuously involve trial and error but over time, you will find what works best for you and your patients.
Discussing PrEP naturally requires very sensitive information to be shared. A mindful and patient-centered approach is necessary to instill the comfort and build proper rapport. In this section, we will review gender-related terminology and how to effectively obtain a sexual and substance use history. Tailoring your approach will continuously involve trial and error but over time, you will find what works best for you and your patients.
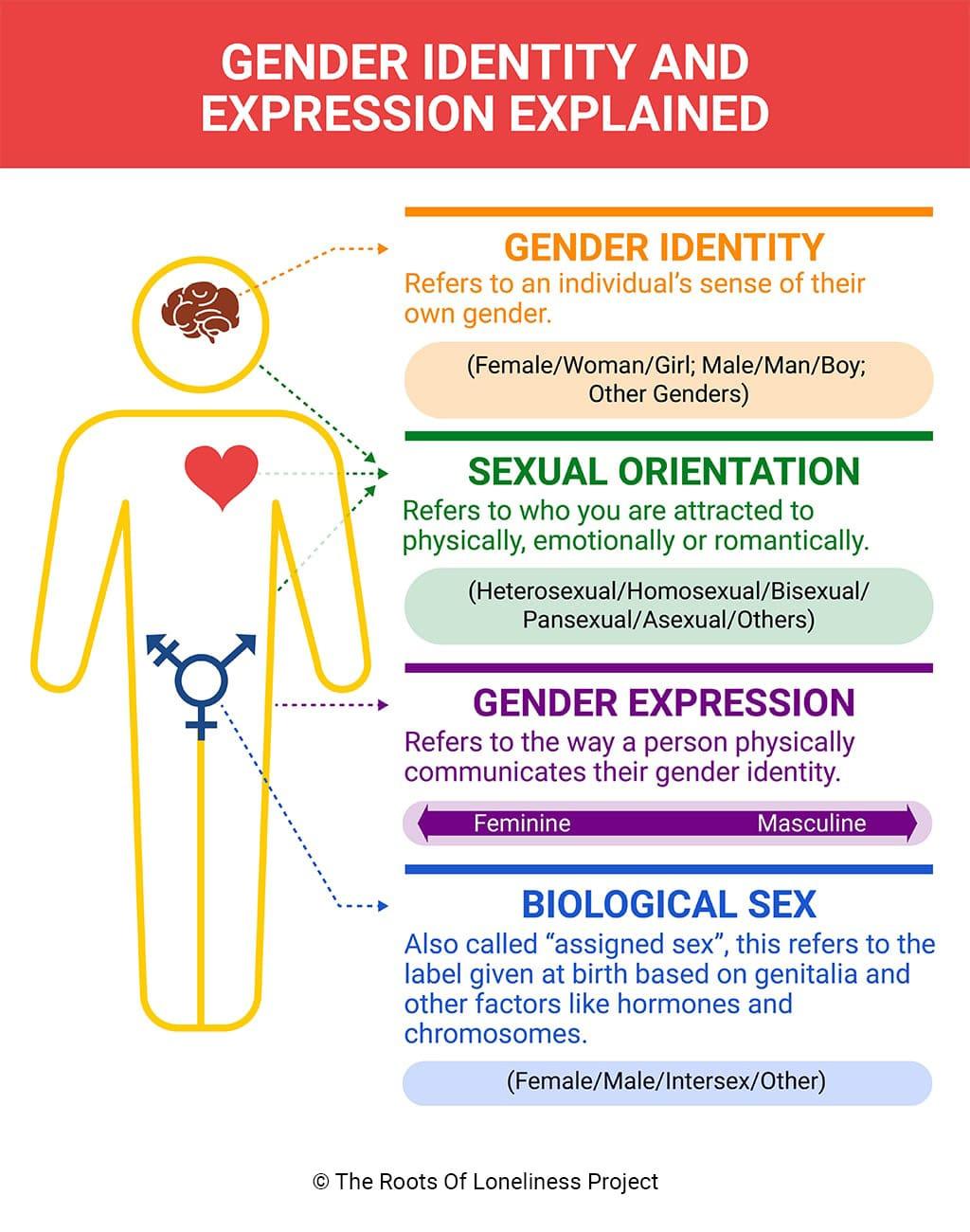
Review of gender-related terminology
Currently, there are different indications of which PrEP medication to use depending on the sex of the patient. Sex is not the same as gender, nor is it equivalent to, or indicative of, someone’s current anatomy, sexual orientation, or sexual practices. It is easy to confuse these terms which can affect internal biases, patient rapport, and overall quality of care. Therefore, it is worthwhile at this point to review gender-related terminology.
The term sex is used to refer to the designation that is given at birth based on biological factors such as sex chromosomes and genitalia present at birth. Typically, a person is assigned male at birth (AMAB) or female at birth (AFAB). A person may also be assigned intersex when there are conditions that make this determination ambiguous (e.g., congenital adrenal hyperplasia, androgen insensitivity syndrome, etc.).
There exist social constructs that are tied to a person’s sex which are sometimes called gender norms. These norms encompass things such as roles and expressions. For example, men have to be in positions of power or only women can wear makeup and dresses. An individual may not always identify or agree with these norms or their bimodality.
Gender identity refers to an individual's internal perception of where in the spectrum of gender norms they fit in most. Relatedly, gender expression is the external presentation of this concept - how a person chooses to look.
Someone whose gender identity is congruent with their sex is termed cisgender.
Someone whose gender identity is different from their sex is termed transgender.
A person whose sex is assigned male at birth (AMAB) but identifies as a female is termed a transgender woman (TGW), sometimes referred to as male-to-female (MtF).
Conversely, there are also female-to-male (FtM) transgender persons.
Sometimes, a person may choose to undergo medical therapies and interventions to affirm their gender identity. This may be referred to as transsexual - a subset of the transgender umbrella term. It is never appropriate to assume a patient’s existing anatomy based on their sex or gender.
Click image for full size view
Click the title of the figure to view source.
Figure 10 - Gender Identity and Expressions
Source: Gender identity and how understanding it can ease loneliness. (n.d.). The Roots of Loneliness Project. https://www.rootsofloneliness.com/gender-identity-loneliness
Sexual orientation refers to the physical, romantic, and/or emotional attraction one feels towards another gender(s).
Heterosexuality is the attraction between those of the opposite sex (i.e. straight)
Homosexuality is the attraction between those of the same sex (i.e. lesbian, gay)
A term often used in medicine is men who have sex with men (MSM).
Bisexuality refers to the attraction to those of both sexes. Asexuality is the lack of attraction to both sexes.
Lastly, sexual practices specifically discuss the sexual acts a person engages in.
Some examples include receptive anal intercourse (RAI), insertive anal intercourse (IAI), receptive vaginal intercourse (RVI), insertive vaginal intercourse (IVI), oral sex, masturbation, etc. Similarly, it is never safe to assume the sexual practices of a patient based on sex, gender, or orientation. We will soon discuss how to take a proper sexual (and substance use) history.
For the purposes of PrEP, an important patient population has been defined based on sex and sexual orientation: men who have sex with men and transgender women who have sex with men (MSM/TGWSM). This will be utilized throughout the toolkit, so please dedicate the time needed to understand these definitions.
This is by no means a comprehensive overview of this topic. This section aims to lay a foundation for understanding some of the nuances of PrEP care that will soon be addressed. For a more comprehensive overview of gender-related terminology and definitions: https://transcare.ucsf.edu/guidelines/terminology.
Sexual and substance use history
The CDC created a helpful framework for taking a sexual history termed “The 5 P’s”. (6) The National Coalition for Sexual Health expanded this to “The 6 P’s” which includes a “Plus” category that encompasses topics such as pleasure, problems, and pride. (7). This coalition also created a helpful video series to improve your approach when taking a sexual history. (8)
National Coalition for Sexual Health. (2022). A new approach to sexual history taking: a video series. Altarum Institute.
https://nationalcoalitionforsexualhealth.org/tools/for-healthcare-providers/video-series
National Coalition for Sexual Health. (2022). Sexual health and your patients: a provider’s guide. Altarum Institute
https://nationalcoalitionforsexualhealth.org/tools/for-healthcare-providers/asset/Provider-Guide_May-2022.pdf
Centers for Disease Control and Prevention. (n.d.). A guide to taking a sexual history. U.S. Department of Health and Human Services.
https://www.cdc.gov/std/treatment/SexualHistory.pdf
The 6 P’s of a Sexual History
- Partners
- Practices
- Past History of STIs
- Protection
- Pregnancy
- Plus: Pleasure, Problems, & Pride
Given the rising incidence rate of HIV among people who inject drugs (PWIDs), it is important to expand on the second “P” in this framework: Practices.
Although this approach raises the question of drug and alcohol use in the context of sex, a simple follow-up question to further assess these habits could be…
“Have you ever used injection drugs that were not prescribed by a doctor such as heroin, cocaine, meth, or others?”
If the patient answers “yes,” ask further about the frequency of use and most recent use. Most importantly, ask if they ever share drug injecting equipment of any sort. This includes needles, syringes, cookers, filters, etc. A common misconception is that the patient is not at risk for acquiring transmissible infections as long as different injection needles are used. Ask carefully and provide risk reduction strategies when applicable.
Relatedly, bloodborne infections such as HIV, Hepatitis B, and Hepatitis C can also be transmitted through needles used for tattoos, particularly in correctional facilities. (42) Inquire about any tattoos the patient has or plans on getting to tailor your care and patient education.
Westergaard, R. P., Spaulding, A. C., & Flanigan, T. P. (2013). HIV among persons incarcerated in the USA: a review of evolving concepts in testing, treatment, and linkage to community care. Current opinion in infectious diseases, 26(1), 10–16.
https://doi.org/10.1097/QCO.0b013e32835c1dd0
It may not always be feasible to ask every question mentioned in this section; the point of this framework is to help you progressively improve your history-taking skills. Remember that it may take several conversations about PrEP before a patient decides that it is right for them. Even if typical risk factors are not made apparent, it is always worthwhile to at least ask your patients if they have heard of PrEP. A patient will never take something into consideration if they don’t have an awareness of it at all.
Uplifting BIPOC for PrEP Uptake
- Providing PrEP to BIPOC
- Barriers to PrEP Faced by
Communities Disproportionately Impacted by HIV - Why Are We Doing So Poorly?
- What providers can do to
improvePrEP utilization
among BIPOC people?
Providing PrEP to BIPOC
The BIPOC acronym stands for Black, Indigenous and People of Color. The term BIPOC is used to highlight the unique relationship to whiteness that Indigenous and Black people have that shapes the experiences of and relationship to white supremacy for all people of color within a U.S. context. Using the term BIPOC is people-first and allows us to shift away from terms like “marginalized” and “minority.”
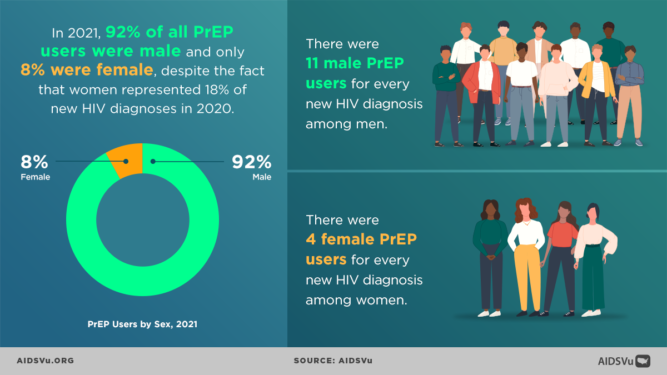
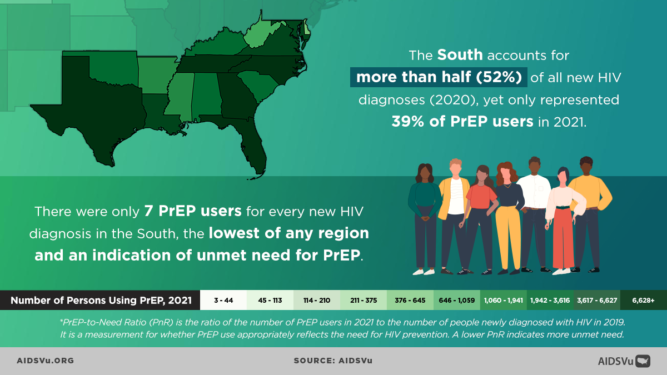
Structural and political determinants of health drive inequities, HIV infection and the use of PrEP among BIPOC. In 2018, at the Conference on Retroviruses and Opportunistic Infections (CROI) new data about PrEP use showed that PrEP medication is not reaching most Americans who could benefit, especially people of color. Out of 500,000 African Americans and nearly 300,000 Latinos across the nation that could benefit from PrEP only 7,000 prescriptions were filled by these communities. This data shows a stark unmet need for HIV prevention medication.

Longstanding social and economic inequities elevate health risks and vulnerabilities for BIPOC, and HIV continues to disproportionately impact these communities, specifically sexual minority men and transgender women in the United States. Disparities are most prominent for Black and Hispanic/Latino groups. At the current rate of infection, estimates suggest that 1 in 2 Black men who have sex with men (MSM) and 1 in 5 Hispanic/Latino MSM will be diagnosed with HIV in their lifetime; these racial and ethnic disparities in HIV prevalence also extend to Black and Hispanic/Latina transgender women. These communities carry a higher burden of HIV compared to white transgender women. In the U.S., an estimated 44% of Black and 26% of Latino/Hispanic transgender women are living with HIV compared to 7% of white transgender women. The substantial disparity of HIV in these communities is driven by experiences of discrimination and stigma, lack of access to healthcare services, lack of education and employment opportunities, and unstable housing.
HIV pre-exposure prophylaxis (PrEP) inequities in the US – both in terms of race and geographical location – have not only persisted in the last decade, but have increased, according to research presented to the 24th International AIDS Conference (AIDS 2022) by Dr Patrick Sullivan from Emory University.
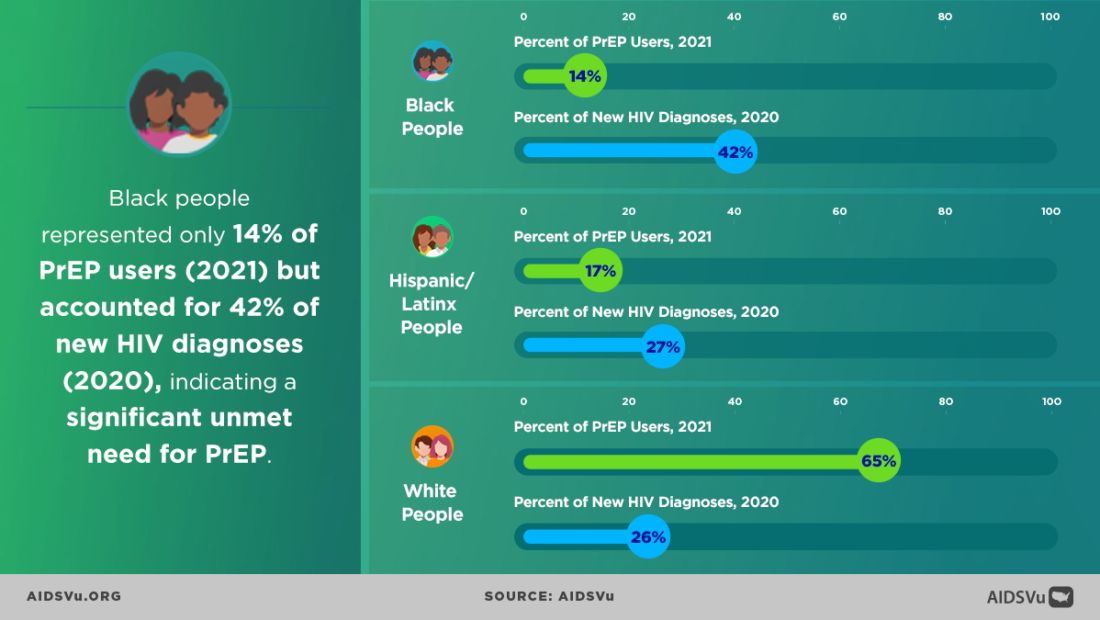
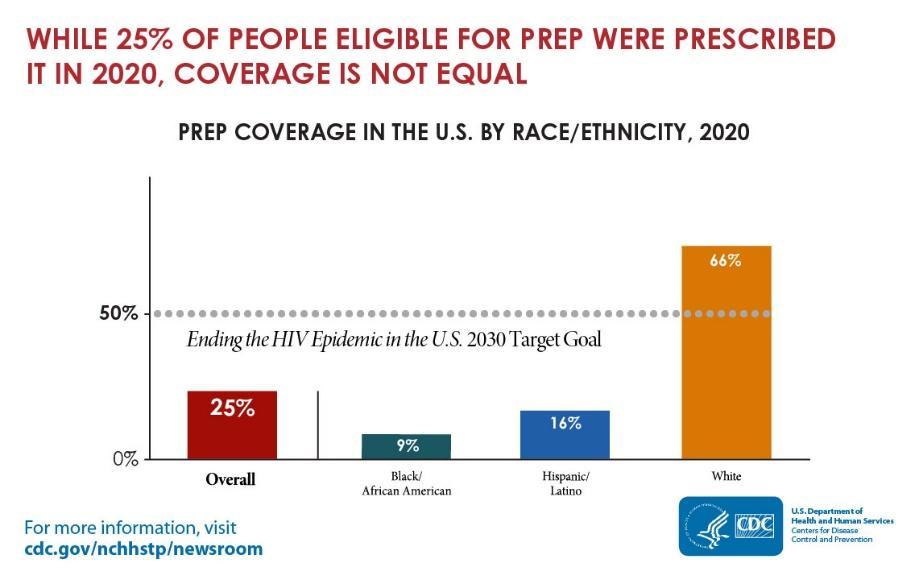
While HIV prevention methods, such as PrEP, are highly effective and necessary in ending the HIV epidemic, serious racial/ethnic disparities, structural inequities, and systemic racism block progress for Black and Hispanic/Latino cis-gender sexual minority men and transgender women in accessing this effective HIV prevention medication. In 2018, at the Conference on Retroviruses and Opportunistic Infections (CROI), new data about PrEP use showed that PrEP medication is not reaching most Americans who could benefit, especially people of color. PrEP uptake has been persistently low among US women, particularly Black cisgender women, who account for 61% of new HIV diagnoses among cis women. Preliminary 2020 CDC data show only 9% (42,372) of the nearly 469,000 Black people who could benefit from PrEP received a prescription in 2020, and only 16% (48,838) of the nearly 313,000 Hispanic/Latino people who could benefit from PrEP received a prescription. Meanwhile, 66% of their White counterparts are utilizing PrEP. This emphasizes the continued unmet need for HIV prevention medication, especially among BIPOC.
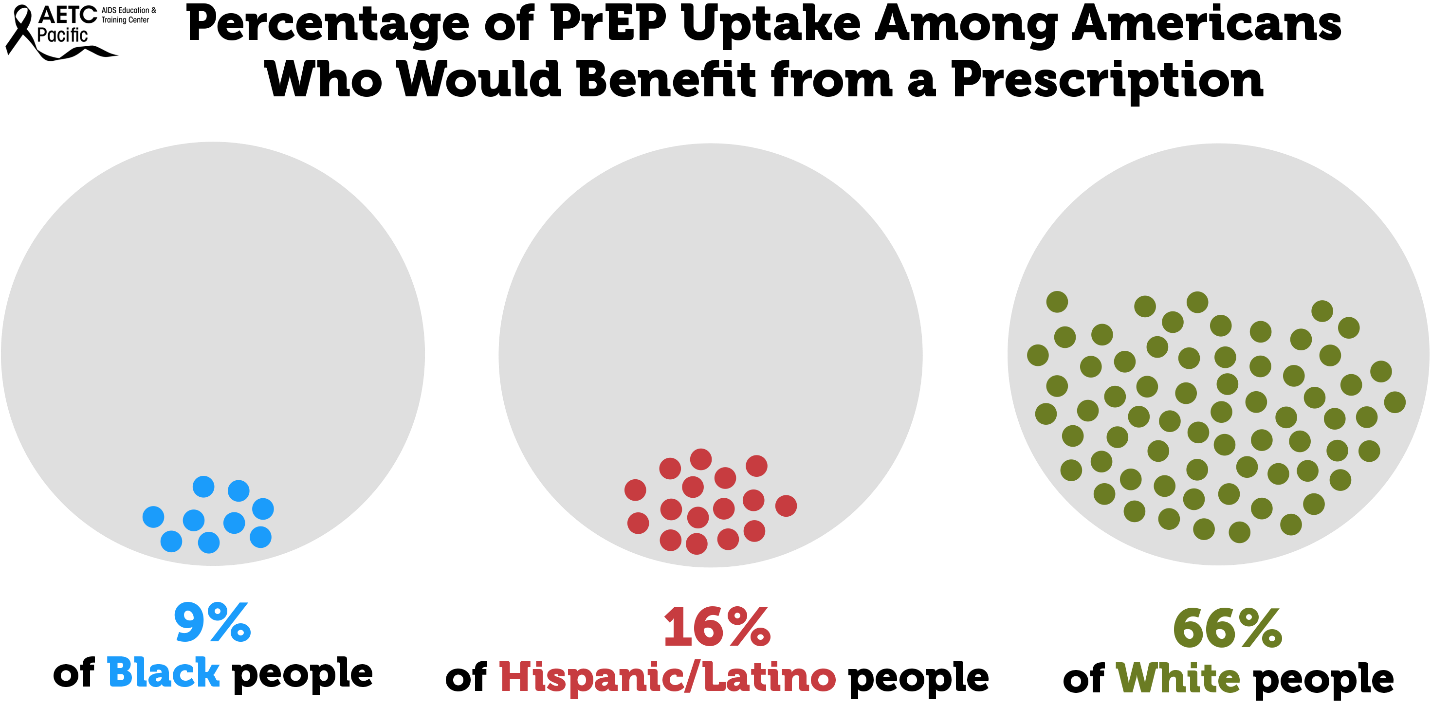
Barriers to PrEP Faced by Communities Disproportionately Impacted by HIV
While African Americans only make up 13% of the U.S. population, they accounted for 42% of the 37,832 new HIV diagnoses in 2018, the highest rate among all racial and ethnic groups. Black women accounted for 57% of the new HIV diagnoses among women. Most new HIV diagnoses among women are attributed to heterosexual contact (84%) followed by injection drug use (16%). Black/African American cisgender women continue to be disproportionately affected by HIV. Challenges to successful PrEP implementation among ciswomen include lack of awareness, access to healthcare, insurance and out-of-pocket costs and low perceived risk.
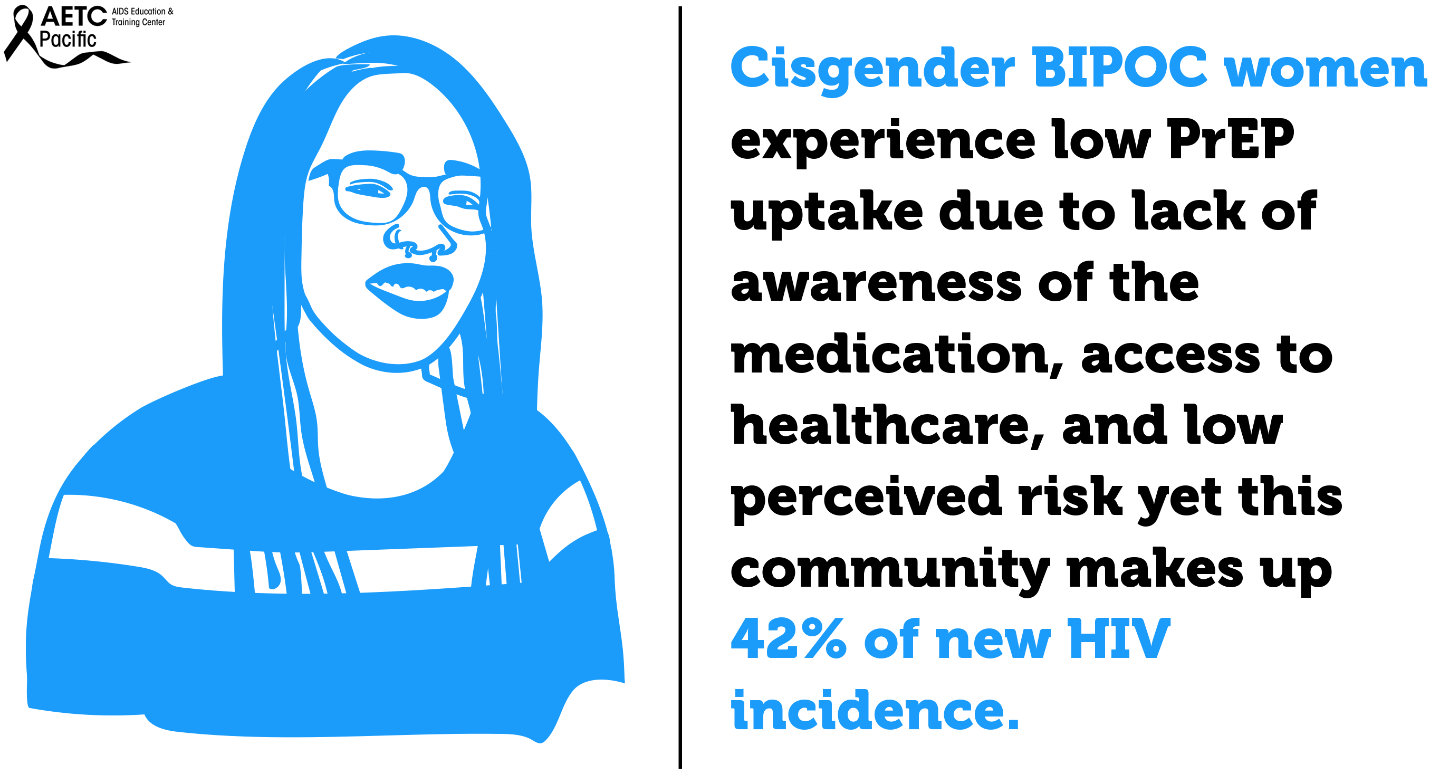

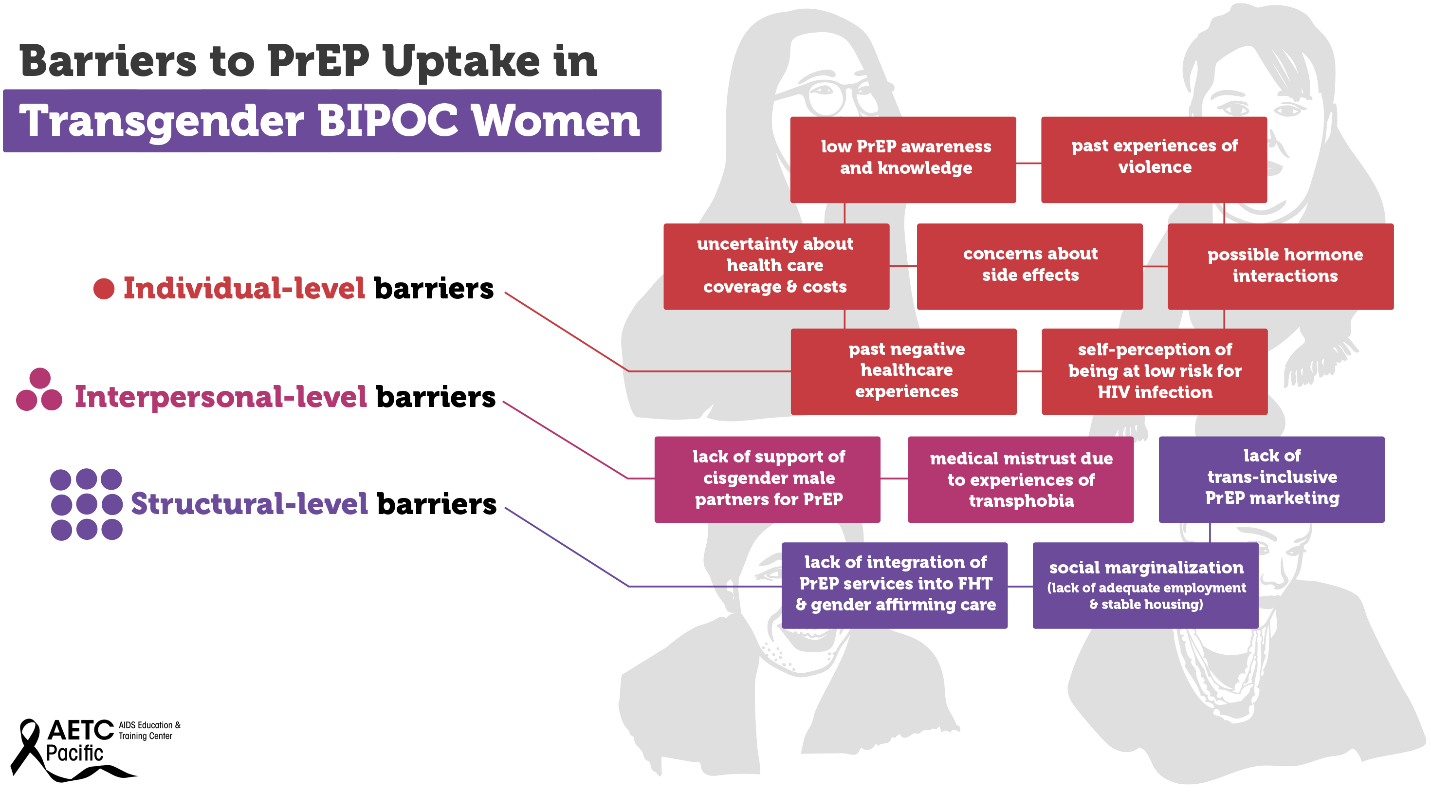
Transgender women—those who were assigned male at birth and who identity as female—are disproportionately affected by the HIV epidemic in the U.S. Between 2009 and 2014, an estimated 1,974 transgender women were diagnosed with HIV in the U.S., representing 84% of transgender people diagnosed. Racial and ethnic minority transgender women bear a higher burden of HIV compared to White transgender women. In the U.S., an estimated 44% of Black transgender women and 26% of Hispanic/Latina transgender women are living with HIV compared to 7% of White transgender women. Barriers to PrEP uptake among transgender women fall along multiple socio-ecological levels. Individual-level barriers may include low PrEP awareness and knowledge, past experiences of violence, uncertainty about health insurance coverage and associated health costs, concerns about side effects, possible hormone interactions, past negative healthcare experiences, and self-perception of being at low risk for HIV infection.
Some examples of interpersonal-level barriers include lack of support of cisgender male partners for PrEP, and medical mistrust due to experiences of transphobia. Structural-level barriers include lack of trans-inclusive PrEP marketing, lack of integration of PrEP services into feminizing hormone therapy (FHT) and gender affirming care, and social marginalization (lack of adequate employment and stable housing). Experiences of social marginalization because of gender, class, race, sexual identity, poverty, and the intersection of these identities and experiences may contribute to increased vulnerability to HIV infection among Black and Hispanic/Latina transgender women. Although several studies have investigated barriers and facilitators to PrEP uptake among transgender women, there is a dearth of research on multilevel barriers to PrEP uptake and adherence specifically among Black and Hispanic/Latinx transgender women.
>
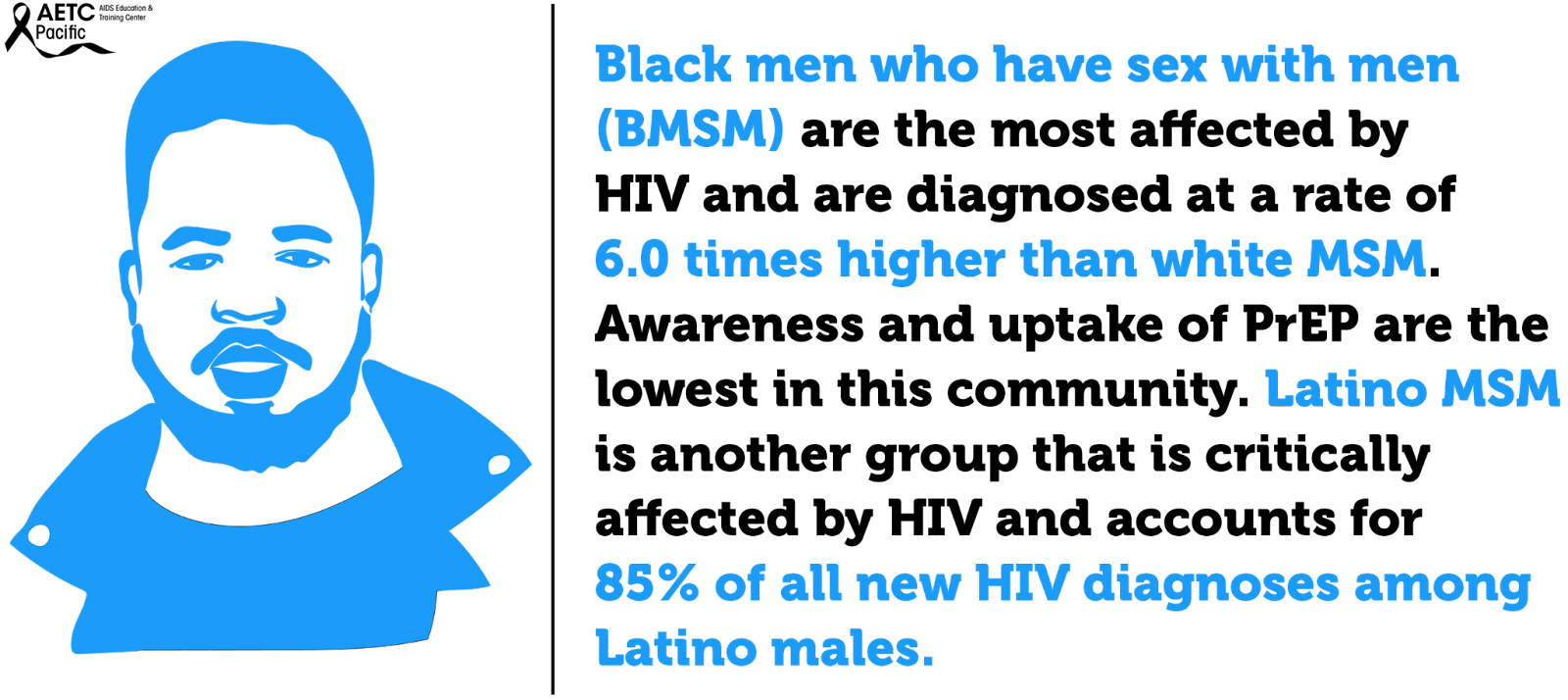
In the United States, Black men who have sex with men (BMSM) are the most affected by HIV and are diagnosed at a rate of 6.0 times higher than white MSM. Awareness and uptake of PrEP are lowest among this community. Latino MSM are another group that is critically affected by HIV and account for 85% of all new HIV diagnoses among Latino males. Like BMSM, barriers like cost and lack of insurance, concerns about side effects, societal or contextual factors such as racism and homophobia and HIV/AIDS conspiracy beliefs are barriers preventing Latino MSM from accessing PrEP.
Youth continue to be a population impacted by higher rates of HIV. In 2019, 21% of new HIV diagnoses were among young people aged 13-24, and most of these cases are among Black and Latino youth. PrEP uptake among racial and ethnic minority adolescents has been slow due to lack of awareness of PrEP, behavioral factors, and legal and financial barriers to adolescents seeking sexual and reproductive health care.


Indian/Alaska Native adults and adolescents increased. Access to PrEP and PEP in American Indian and Alaska Native (AI/AN) communities can be limited and there are cultural and historical considerations that impact Tribal communities’ ability to adopt PrEP, and social and systematic considerations that also present challenges.
According to the CDC, Asian Americans who make up only 6 percent of the U.S. population, make up 2 percent of all HIV infections. The CDC suggests this disparity is so great due to cultural factors, such as language barriers and immigration issues that create barriers to accessing healthcare (though this only addresses access to care for Asian immigrants, not Asian Americans who have lived in the U.S. for generations.) Low PrEP utilization rates among the Asian/Pacific Islander communities could be because of lack of awareness about the medication, misconceptions regarding its affordability, misinformation about its effects and fear of community stigma about sex.


HIV incidence is high among people who use drugs and are unhoused across the U.S. This is fueled by the opioid epidemic, lack of affordable housing, socioeconomic marginalization, and other complex factors. Despite this, PrEP is largely under-utilized among this vulnerable population.
Some of the low PrEP uptake can be attributed to practical barriers, such as competing health and survival needs, and medications being lost or stolen due to being unhoused and without a safe place to store medications. However, there is also a pervasive belief among clinicians that people who use drugs who are unhoused are not good candidates for PrEP. This idea is likely due to overlapping stigmas and isn’t supported by data.
According to California Department of Public Health Office of AIDS, Substance Use Community engagement efforts have linked methamphetamine use to increased risk for HIV infection and falling out of medical care. County-specific data on methamphetamine use is not easily accessible. This CDPH report on Ending the HIV Epidemic utilizes county-specific data on 1) amphetamine-related Emergency Department (ED) visits and 2) amphetamine-related deaths 2011-2018; all data obtained from the California Opioid Overdose Surveillance Dashboard. From 2011-2013, there was an overall increase of ED visits attributable to amphetamine use in Alameda County, from 5.01 visits/100,000 population in 2011, to 5.81/100,000 in 2013. Since 2013, ED visits steadily declined to a low of 1.7/100,000 in 2018. Deaths, on the other hand, fluctuated more widely, from a low of 1.18/100,000 in 2011 to a high of 4/100,000 in 2013.
However, in 2018, deaths attributable to amphetamine use spiked dramatically to a high of 8.26/100,000. This spike, coming as ED visits continued to decline, may reflect persons not seeking medical care for amphetamine overdose rather than decreased use. Not seeking medical care for amphetamine overdose could be secondary to a lack of access to medical care, fear of legal ramifications, or to unwitnessed overdose— the reasons are unclear from this data.
Why Are We Doing So Poorly?
National HIV screening recommendations place healthcare providers at the helm in the fight against HIV. While the goal of healthcare providers should be to ensure high quality HIV prevention for patients, their stigmatizing attitudes, beliefs, and behaviors toward vulnerable populations impede progress in identifying patients who would benefit from PrEP. Some of the assumptions that clinicians make about patients seeking PrEP accompany and may exacerbate systemic factors affecting PrEP uptake, like patients’ distrust, lack of access to care, and lack of awareness of PrEP. One of the key reasons cited for clinicians not prescribing PrEP is their assumption that patients will not be adherent to PrEP followed by assumptions that patients taking PrEP will increase their frequency of condomless sex or number of sexual partners.
What is Medical Mistrust?
Medical Mistrust is “an absence of trust that health care providers and organizations genuinely care for patients’ interests, are honest, practice confidentiality, and have the competence to produce the best possible results.”
The Origins of Medical Mistrust
Research indicates that Medical Mistrust is rooted in the history of mistreatment, discrimination, and abuse perpetrated against Black and brown bodies, sexual and gender diverse people, and other marginalized groups by both the State and the medical establishment. In addition, contemporary experiences of discrimination in health such as inequities in access to medical insurance, health care facilities, and treatments along with institutional practices that make it difficult for marginalized communities to access quality health care also play a significant role in shaping Medical Mistrust particularly among Black and other patients of color. Studies that have explored healthcare experiences among different populations have identified a significant association between race and medical mistrust such that Black Americans are significantly more likely than White Americans to mistrust healthcare personnel and the medical system as a whole.

The Impact of Medical Mistrust
- Trust in health care among Americans has declined in recent decades
- Mistrust may prevent people from seeking care
- Black Americans are more likely than whites to say they do not trust their medical provider
- People who say they mistrust health care organizations are less likely to take medical advice, keep follow-up appointments, or fill prescriptions
- People who say they mistrust health care systems are more likely to report being in poor health
Talking with Patients about Medical Mistrust – How to Have Those Conversations
In the U.S., trust in the health care system is extremely low. Reversing medical mistrust is integral to building and strengthening patient-provider relationships. Health care professionals should assume patient mistrust and take the appropriate steps to address it.
Effective ways to communicate with patients about Medical Mistrust
- Openly discuss mistrust with patients
- Talk with, not at patients
- Partner with patients in making decisions about their health care
Medical mistrust has been identified as a primary driver of racial and ethnic inequities in health outcomes in the U.S. Among Black individuals, medical mistrust stems from experiences with discrimination in healthcare and is the result of persistent social inequity and structural racism, both contemporary and historical. Regarding PrEP use, several studies have identified medical mistrust as a barrier to uptake among Black individuals. Studies that have explored healthcare experiences among different populations have identified a significant association between race and medical mistrust such that Black Americans are significantly more likely than White Americans to mistrust healthcare personnel and the medical system as a whole.
Medical mistrust is a major barrier to a strong patient-clinician relationship. Patient mistrust in health care clinicians and in the health care system generally, negatively influences patient behavior and health outcomes.
Furthermore, the staff of healthcare organizations do not reflect the face of the communities they serve. Numerous studies have shown that increased provider diversity is associated with improved healthcare quality. Concordant care, defined as a patient and provider sharing a common attribute such as race, ethnicity, or gender, has been associated with improved quality of care. According to the Association of American Medical Colleges, only about 36% of active physicians are female, and only about 5% of physicians identify as Black or African-American, despite this group making up 13% of the U.S. population. Fewer than 6% of physicians identify as Hispanic, despite Hispanics making up about 19% of the U.S. population. However, 28% of physicians and surgeons in the United States are immigrants, with doctors from India and China making up the largest groups. This speaks to issues of systemic oppression: People from minority groups that have been oppressed for generations in the United States are less represented as physicians than are immigrants of color. There is a long history related to how we have gotten here. Since Abraham Flexner’s review of the American medical education system in 1910, it was believed that there was a limited role for Black physicians in the medical community and that Black physicians possessed less potential and ability than their White counterparts. The result of this characterization led to the closure of five of seven African American medical schools. The impact is still felt today.
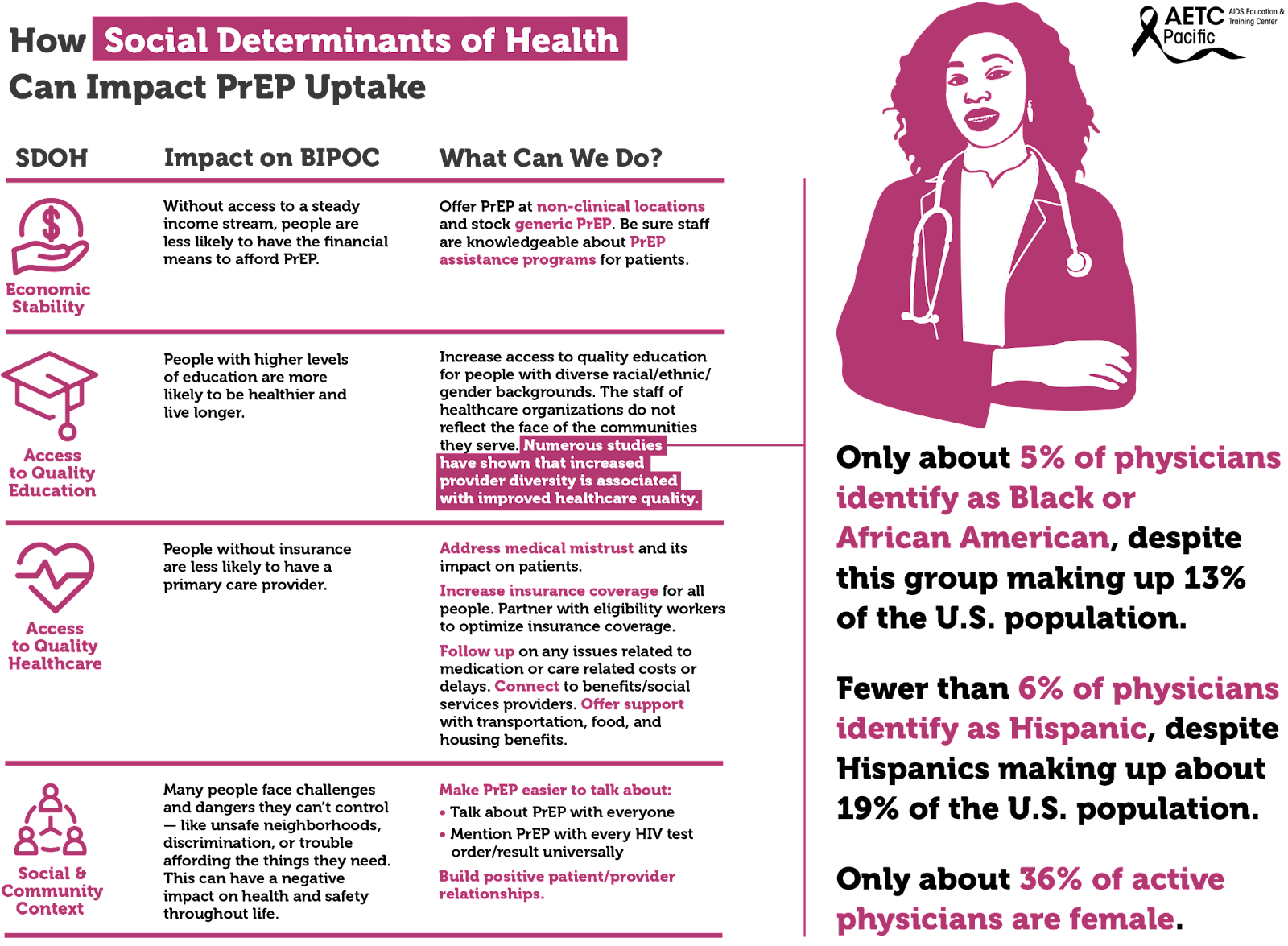
Social determinants of health, the conditions in environments where people live, drive inequities, HIV acquisition and the use of PrEP among BIPOC. These social determinants include, among others, economic stability, access to quality education, and access to quality healthcare.
The uptake of PrEP has been shown to be impacted by unmet social determinants of health (SDOH) needs. These include basic needs (e.g., food, shelter, water), health/social service needs (e.g., healthcare), and economic needs (e.g., money for savings). Socioeconomic factors identified to affect PrEP uptake included lack of insurance, difficult access to transportation, and inflexible work situations. Individuals with more unmet SDOH needs may have other priorities that divert their focus from obtaining preventative health services.
There are multiple socioeconomic factors to consider when looking at the barriers to PrEP uptake among disproportionately impacted communities at both the individual-level and structural-level barriers. Individual-level barriers to PrEP uptake include low PrEP awareness and knowledge, past experiences of violence, uncertainty about health insurance coverage and associated health costs, concerns about side effects, possible hormone interactions, past negative healthcare experiences, and self-perception of being at low risk for HIV infection.
What providers can do to improve PrEP utilization among BIPOC people?
- Prior to meeting your patient:
- Check your bias and personal story: what baggage do you bring to the table?
- Look at their chart to get a sense of who they are
- Beware of microaggressions
- Don’t speak in generalities
- Don’t assume race/ethnicity
- Don’t assume gender/sexual identity
- Don’t assume pop culture references
- Condescension while being questioned
- What to bring to the patient interaction?
- Any member of the care team (e.g., PrEP Navigator, Case Manager) can learn about the familial context and/or patient’s culture and share what they learn with the provider and/or providers can build rapport by expressing an interest in learning about these aspects of the patient's life as they get to know each other
- Questions around culture
- Familial Context
- Learn about the familial responsibilities of the patient
- Learn about the patient’s biological/chosen support system
- Ask about personal & familial experience with health insurance
- Ensure prescriptions are affordable within the patient’s budget
- Discuss ease of getting to your clinic
- Discuss ease of physically accessing referral care and/or pharmacies
- Words Matter
- Providers can utilize a mutually agreed-upon opening script within the clinic about how to frame these questions. An example could be: "I'm interested in learning about how we can support you now or in the future" or "it's important to me to know if there are any issues that are coming up even if I can't help right away"
- It is not “dumbing-down” to speak to patients in a culturally-responsive manner
- If you do not speak their vernacular, learn
- If you misspeak & offend, apologize
- Watch for “Why” questions: instead of asking “Why do you want to take PrEP?” or “why don’t you use condoms,” you can ask “Tell me about what makes you want to take PrEP” or “Tell me about how you make decisions about condoms”
- Do not ask: “Do you understand?”
- Ask: “Please tell me what you think I said. It is important that we are on the same page.”
- Support Resilience
- It is important to “reframe the misconception to see [Black LGBTQ people] as “at risk” and instead see them as “at promise” for a future of good health and well-being anchored in their own resilience and supported by our abilities as staff, providers, and institutions to provide services to contribute to their resilience.”
- Consider exploring strengths and coping strategies by asking: What do you love about yourself? What has helped you to keep going even when times are hard? How can I best help you as you start PrEP?
- Care to Treatment
- Take time to address fears about a particular drug, pharmaceutical company, or the healthcare industrial complex. Take care not to argue a point, but rather to hear their perspective/concerns, then ask for permission to share additional information or your experience as a clinician, if appropriate.
- Storytelling of other patients’ experiences with medication is key for trust
- Share personal experience when appropriate, this is critical when recommending preventative care
- Be transparent if you or your clinic have been involved with any clinical trials or are receiving funding from pharmaceutical companies
- Create a safe & welcoming clinic
- Staff Diversity
- Actively engage clinic leadership to champion Diversity, Equity, Inclusion and Anti-Racism efforts.
- Hire and retain staff that reflect the patient population, especially clinicians
- Encourage clinic leadership (including Human Resources) to adopt workplace policies, procedures, and compensation rates that foster retention of a culturally concordant workforce.
- Speak with staff and conduct quarterly trainings on cultural responsiveness.
- Have inclusive imagery and signs visible.
- Have BIPOC-centric brochures & magazines in waiting areas
- Post a Diversity & Inclusion statement in a conspicuous location
- Bringing them back to care
- Before the patient leaves the visit:
- Confirm the best form of future contact
- If you have a portal, make sure that it is on their mobile phone
- Ask them to send you a Test Message
- Introduce them to the person(s) who may follow-up
- Patients, clients, customers may come once, but maybe not again
- Look at your client-level data and see if your BIPOC clients are coming back for their appointments
- It’s probably not them … It may just be you (and/or your staff)
- Conduct an anonymous survey of patients
- Be committed to receiving feedback and change
- Reiterate that you are committed to their success
- Ask for a verbal report card before the patient leaves
Address Social Determinants of Health whenever possible. One of the findings in a PrEP navigation study highlights the benefit of offering clinical and non-clinical PrEP services to clients in one place which facilitates clients’ access to PrEP services. A co-located approach can ameliorate barriers such as time constraints, PrEP cost and lack of insurance, and low/limited income. This is particularly important when there is a low PrEP uptake among racial and gender minorities who are disproportionately affected by HIV.
- Hire a PrEP Navigator or train community health workers in PrEP Navigation
- Partner with eligibility workers to optimize insurance coverage
- Support with transportation benefits
- Connect to food and housing resources
- Connect to benefits/social services providers
- Follow up on any issues related to medication or care related costs or delays
Make PrEP easier to talk about:
- Talk about PrEP with everyone.
- Mention PrEP with every HIV test order/result universally.
- Make PrEP easier for patients to get:
- Stock generic PrEP and use it as first line agent (cost-effective).
- Offer tele-prep follow-up as an option.
- Do not withhold next prescriptions due to missed appointment (as long as the HIV test is complete and negative).
Offer options:
- Consider offering a 1-month follow-up if the patient is ambivalent or unsure about adherence (before extending to every 3 month visits).
- Honor choice: Continue to offer all tools in the HIV prevention toolkit even if it’s not the provider’s preference.
- If patient is not ready for sexual history discussion on first visit, that’s ok. Revisit this discussion at next appointment or when rapport is built.
- Greater than AIDS PrEP campaign “Powered by PrEP”: POC centered messaging including testimonials in English and Spanish
- REACH LA’s WAP campaign
- Black AIDS Institute: black cis women
- Cardea: HIV Care for Native patients
- Labeling transwomen as MSM
- MSM of color - Leo Moore presentation
- People of trans experience - evidence of lack of interaction with gender-affirming hormones
- Reproductive health - evidence of safety in conceiving, pregnancy & lactation. (HIVE toolkit)
- Seicus PrEP Education for Youth-Serving Primary Care Providers Toolkit
- Lack of diversity in healthcare Infographic
- National LGBT Health Education Center: “Understanding and Addressing the Social Determinants of Health for Black LGBTQ People”
- Same-day Oral PrEP quick guide: https://www.ebgtz.org/resource/same-day-prep/
Barriers to PrEP Faced by Communities Disproportionately Impacted by HIV
While African Americans only make up 13% of the U.S. population, they accounted for 42% of the 37,832 new HIV diagnoses in 2018, the highest rate among all racial and ethnic groups. Black women accounted for 57% of the new HIV diagnoses among women. Most new HIV diagnoses among women are attributed to heterosexual contact (84%) followed by injection drug use (16%). Black/African American cisgender women continue to be disproportionately affected by HIV. Challenges to successful PrEP implementation among ciswomen include lack of awareness, access to healthcare, insurance and out-of-pocket costs and low perceived risk.


Transgender women—those who were assigned male at birth and who identity as female—are disproportionately affected by the HIV epidemic in the U.S. Between 2009 and 2014, an estimated 1,974 transgender women were diagnosed with HIV in the U.S., representing 84% of transgender people diagnosed. Racial and ethnic minority transgender women bear a higher burden of HIV compared to White transgender women. In the U.S., an estimated 44% of Black transgender women and 26% of Hispanic/Latina transgender women are living with HIV compared to 7% of White transgender women. Barriers to PrEP uptake among transgender women fall along multiple socio-ecological levels. Individual-level barriers may include low PrEP awareness and knowledge, past experiences of violence, uncertainty about health insurance coverage and associated health costs, concerns about side effects, possible hormone interactions, past negative healthcare experiences, and self-perception of being at low risk for HIV infection.
Some examples of interpersonal-level barriers include lack of support of cisgender male partners for PrEP, and medical mistrust due to experiences of transphobia. Structural-level barriers include lack of trans-inclusive PrEP marketing, lack of integration of PrEP services into feminizing hormone therapy (FHT) and gender affirming care, and social marginalization (lack of adequate employment and stable housing). Experiences of social marginalization because of gender, class, race, sexual identity, poverty, and the intersection of these identities and experiences may contribute to increased vulnerability to HIV infection among Black and Hispanic/Latina transgender women. Although several studies have investigated barriers and facilitators to PrEP uptake among transgender women, there is a dearth of research on multilevel barriers to PrEP uptake and adherence specifically among Black and Hispanic/Latinx transgender women.
>

In the United States, Black men who have sex with men (BMSM) are the most affected by HIV and are diagnosed at a rate of 6.0 times higher than white MSM. Awareness and uptake of PrEP are lowest among this community. Latino MSM are another group that is critically affected by HIV and account for 85% of all new HIV diagnoses among Latino males. Like BMSM, barriers like cost and lack of insurance, concerns about side effects, societal or contextual factors such as racism and homophobia and HIV/AIDS conspiracy beliefs are barriers preventing Latino MSM from accessing PrEP.
Youth continue to be a population impacted by higher rates of HIV. In 2019, 21% of new HIV diagnoses were among young people aged 13-24, and most of these cases are among Black and Latino youth. PrEP uptake among racial and ethnic minority adolescents has been slow due to lack of awareness of PrEP, behavioral factors, and legal and financial barriers to adolescents seeking sexual and reproductive health care.


Indian/Alaska Native adults and adolescents increased. Access to PrEP and PEP in American Indian and Alaska Native (AI/AN) communities can be limited and there are cultural and historical considerations that impact Tribal communities’ ability to adopt PrEP, and social and systematic considerations that also present challenges.
According to the CDC, Asian Americans who make up only 6 percent of the U.S. population, make up 2 percent of all HIV infections. The CDC suggests this disparity is so great due to cultural factors, such as language barriers and immigration issues that create barriers to accessing healthcare (though this only addresses access to care for Asian immigrants, not Asian Americans who have lived in the U.S. for generations.) Low PrEP utilization rates among the Asian/Pacific Islander communities could be because of lack of awareness about the medication, misconceptions regarding its affordability, misinformation about its effects and fear of community stigma about sex.


HIV incidence is high among people who use drugs and are unhoused across the U.S. This is fueled by the opioid epidemic, lack of affordable housing, socioeconomic marginalization, and other complex factors. Despite this, PrEP is largely under-utilized among this vulnerable population.
Some of the low PrEP uptake can be attributed to practical barriers, such as competing health and survival needs, and medications being lost or stolen due to being unhoused and without a safe place to store medications. However, there is also a pervasive belief among clinicians that people who use drugs who are unhoused are not good candidates for PrEP. This idea is likely due to overlapping stigmas and isn’t supported by data.
According to California Department of Public Health Office of AIDS, Substance Use Community engagement efforts have linked methamphetamine use to increased risk for HIV infection and falling out of medical care. County-specific data on methamphetamine use is not easily accessible. This CDPH report on Ending the HIV Epidemic utilizes county-specific data on 1) amphetamine-related Emergency Department (ED) visits and 2) amphetamine-related deaths 2011-2018; all data obtained from the California Opioid Overdose Surveillance Dashboard. From 2011-2013, there was an overall increase of ED visits attributable to amphetamine use in Alameda County, from 5.01 visits/100,000 population in 2011, to 5.81/100,000 in 2013. Since 2013, ED visits steadily declined to a low of 1.7/100,000 in 2018. Deaths, on the other hand, fluctuated more widely, from a low of 1.18/100,000 in 2011 to a high of 4/100,000 in 2013.
However, in 2018, deaths attributable to amphetamine use spiked dramatically to a high of 8.26/100,000. This spike, coming as ED visits continued to decline, may reflect persons not seeking medical care for amphetamine overdose rather than decreased use. Not seeking medical care for amphetamine overdose could be secondary to a lack of access to medical care, fear of legal ramifications, or to unwitnessed overdose— the reasons are unclear from this data.
Why Are We Doing So Poorly?
National HIV screening recommendations place healthcare providers at the helm in the fight against HIV. While the goal of healthcare providers should be to ensure high quality HIV prevention for patients, their stigmatizing attitudes, beliefs, and behaviors toward vulnerable populations impede progress in identifying patients who would benefit from PrEP. Some of the assumptions that clinicians make about patients seeking PrEP accompany and may exacerbate systemic factors affecting PrEP uptake, like patients’ distrust, lack of access to care, and lack of awareness of PrEP. One of the key reasons cited for clinicians not prescribing PrEP is their assumption that patients will not be adherent to PrEP followed by assumptions that patients taking PrEP will increase their frequency of condomless sex or number of sexual partners.

What is Medical Mistrust?
Medical Mistrust is “an absence of trust that health care providers and organizations genuinely care for patients’ interests, are honest, practice confidentiality, and have the competence to produce the best possible results.”
The Origins of Medical Mistrust
Research indicates that Medical Mistrust is rooted in the history of mistreatment, discrimination, and abuse perpetrated against Black and brown bodies, sexual and gender diverse people, and other marginalized groups by both the State and the medical establishment. In addition, contemporary experiences of discrimination in health such as inequities in access to medical insurance, health care facilities, and treatments along with institutional practices that make it difficult for marginalized communities to access quality health care also play a significant role in shaping Medical Mistrust particularly among Black and other patients of color. Studies that have explored healthcare experiences among different populations have identified a significant association between race and medical mistrust such that Black Americans are significantly more likely than White Americans to mistrust healthcare personnel and the medical system as a whole.

The Impact of Medical Mistrust
- Trust in health care among Americans has declined in recent decades
- Mistrust may prevent people from seeking care
- Black Americans are more likely than whites to say they do not trust their medical provider
- People who say they mistrust health care organizations are less likely to take medical advice, keep follow-up appointments, or fill prescriptions
- People who say they mistrust health care systems are more likely to report being in poor health
Talking with Patients about Medical Mistrust – How to Have Those Conversations
In the U.S., trust in the health care system is extremely low. Reversing medical mistrust is integral to building and strengthening patient-provider relationships. Health care professionals should assume patient mistrust and take the appropriate steps to address it.
Effective ways to communicate with patients about Medical Mistrust
- Openly discuss mistrust with patients
- Talk with, not at patients
- Partner with patients in making decisions about their health care
Medical mistrust has been identified as a primary driver of racial and ethnic inequities in health outcomes in the U.S. Among Black individuals, medical mistrust stems from experiences with discrimination in healthcare and is the result of persistent social inequity and structural racism, both contemporary and historical. Regarding PrEP use, several studies have identified medical mistrust as a barrier to uptake among Black individuals. Studies that have explored healthcare experiences among different populations have identified a significant association between race and medical mistrust such that Black Americans are significantly more likely than White Americans to mistrust healthcare personnel and the medical system as a whole.
Medical mistrust is a major barrier to a strong patient-clinician relationship. Patient mistrust in health care clinicians and in the health care system generally, negatively influences patient behavior and health outcomes.
Furthermore, the staff of healthcare organizations do not reflect the face of the communities they serve. Numerous studies have shown that increased provider diversity is associated with improved healthcare quality. Concordant care, defined as a patient and provider sharing a common attribute such as race, ethnicity, or gender, has been associated with improved quality of care. According to the Association of American Medical Colleges, only about 36% of active physicians are female, and only about 5% of physicians identify as Black or African-American, despite this group making up 13% of the U.S. population. Fewer than 6% of physicians identify as Hispanic, despite Hispanics making up about 19% of the U.S. population. However, 28% of physicians and surgeons in the United States are immigrants, with doctors from India and China making up the largest groups. This speaks to issues of systemic oppression: People from minority groups that have been oppressed for generations in the United States are less represented as physicians than are immigrants of color. There is a long history related to how we have gotten here. Since Abraham Flexner’s review of the American medical education system in 1910, it was believed that there was a limited role for Black physicians in the medical community and that Black physicians possessed less potential and ability than their White counterparts. The result of this characterization led to the closure of five of seven African American medical schools. The impact is still felt today.

Social determinants of health, the conditions in environments where people live, drive inequities, HIV acquisition and the use of PrEP among BIPOC. These social determinants include, among others, economic stability, access to quality education, and access to quality healthcare.
The uptake of PrEP has been shown to be impacted by unmet social determinants of health (SDOH) needs. These include basic needs (e.g., food, shelter, water), health/social service needs (e.g., healthcare), and economic needs (e.g., money for savings). Socioeconomic factors identified to affect PrEP uptake included lack of insurance, difficult access to transportation, and inflexible work situations. Individuals with more unmet SDOH needs may have other priorities that divert their focus from obtaining preventative health services.
There are multiple socioeconomic factors to consider when looking at the barriers to PrEP uptake among disproportionately impacted communities at both the individual-level and structural-level barriers. Individual-level barriers to PrEP uptake include low PrEP awareness and knowledge, past experiences of violence, uncertainty about health insurance coverage and associated health costs, concerns about side effects, possible hormone interactions, past negative healthcare experiences, and self-perception of being at low risk for HIV infection.
What Providers Can Do To Improve PrEP Utilization Among BIPOC People?
- Prior to meeting your patient:
- Check your bias and personal story: what baggage do you bring to the table?
- Look at their chart to get a sense of who they are
- Beware of microaggressions
- Don’t speak in generalities
- Don’t assume race/ethnicity
- Don’t assume gender/sexual identity
- Don’t assume pop culture references
- Condescension while being questioned
- What to bring to the patient interaction?
- Any member of the care team (e.g., PrEP Navigator, Case Manager) can learn about the familial context and/or patient’s culture and share what they learn with the provider and/or providers can build rapport by expressing an interest in learning about these aspects of the patient's life as they get to know each other
- Questions around culture
- Familial Context
- Learn about the familial responsibilities of the patient
- Learn about the patient’s biological/chosen support system
- Ask about personal & familial experience with health insurance
- Ensure prescriptions are affordable within the patient’s budget
- Discuss ease of getting to your clinic
- Discuss ease of physically accessing referral care and/or pharmacies
- Words Matter
- Providers can utilize a mutually agreed-upon opening script within the clinic about how to frame these questions. An example could be: "I'm interested in learning about how we can support you now or in the future" or "it's important to me to know if there are any issues that are coming up even if I can't help right away"
- It is not “dumbing-down” to speak to patients in a culturally-responsive manner
- If you do not speak their vernacular, learn
- If you misspeak & offend, apologize
- Watch for “Why” questions: instead of asking “Why do you want to take PrEP?” or “why don’t you use condoms,” you can ask “Tell me about what makes you want to take PrEP” or “Tell me about how you make decisions about condoms”
- Do not ask: “Do you understand?”
- Ask: “Please tell me what you think I said. It is important that we are on the same page.”
- Support Resilience
- It is important to “reframe the misconception to see [Black LGBTQ people] as “at risk” and instead see them as “at promise” for a future of good health and well-being anchored in their own resilience and supported by our abilities as staff, providers, and institutions to provide services to contribute to their resilience.”
- Consider exploring strengths and coping strategies by asking: What do you love about yourself? What has helped you to keep going even when times are hard? How can I best help you as you start PrEP?
- Care to Treatment
- Take time to address fears about a particular drug, pharmaceutical company, or the healthcare industrial complex. Take care not to argue a point, but rather to hear their perspective/concerns, then ask for permission to share additional information or your experience as a clinician, if appropriate.
- Storytelling of other patients’ experiences with medication is key for trust
- Share personal experience when appropriate, this is critical when recommending preventative care
- Be transparent if you or your clinic have been involved with any clinical trials or are receiving funding from pharmaceutical companies
- Create a safe & welcoming clinic
- Staff Diversity
- Actively engage clinic leadership to champion Diversity, Equity, Inclusion and Anti-Racism efforts.
- Hire and retain staff that reflect the patient population, especially clinicians
- Encourage clinic leadership (including Human Resources) to adopt workplace policies, procedures, and compensation rates that foster retention of a culturally concordant workforce.
- Speak with staff and conduct quarterly trainings on cultural responsiveness.
- Have inclusive imagery and signs visible.
- Have BIPOC-centric brochures & magazines in waiting areas
- Post a Diversity & Inclusion statement in a conspicuous location
- Bringing them back to care
- Before the patient leaves the visit:
- Confirm the best form of future contact
- If you have a portal, make sure that it is on their mobile phone
- Ask them to send you a Test Message
- Introduce them to the person(s) who may follow-up
- Patients, clients, customers may come once, but maybe not again
- Look at your client-level data and see if your BIPOC clients are coming back for their appointments
- It’s probably not them … It may just be you (and/or your staff)
- Conduct an anonymous survey of patients
- Be committed to receiving feedback and change
- Reiterate that you are committed to their success
- Ask for a verbal report card before the patient leaves
Address Social Determinants of Health whenever possible. One of the findings in a PrEP navigation study highlights the benefit of offering clinical and non-clinical PrEP services to clients in one place which facilitates clients’ access to PrEP services. A co-located approach can ameliorate barriers such as time constraints, PrEP cost and lack of insurance, and low/limited income. This is particularly important when there is a low PrEP uptake among racial and gender minorities who are disproportionately affected by HIV.
- Hire a PrEP Navigator or train community health workers in PrEP Navigation
- Partner with eligibility workers to optimize insurance coverage
- Support with transportation benefits
- Connect to food and housing resources
- Connect to benefits/social services providers
- Follow up on any issues related to medication or care related costs or delays
Make PrEP easier to talk about:
- Talk about PrEP with everyone.
- Mention PrEP with every HIV test order/result universally.
- Make PrEP easier for patients to get:
- Stock generic PrEP and use it as first line agent (cost-effective).
- Offer tele-prep follow-up as an option.
- Do not withhold next prescriptions due to missed appointment (as long as the HIV test is complete and negative).
Offer options:
- Consider offering a 1-month follow-up if the patient is ambivalent or unsure about adherence (before extending to every 3 month visits).
- Honor choice: Continue to offer all tools in the HIV prevention toolkit even if it’s not the provider’s preference.
- If patient is not ready for sexual history discussion on first visit, that’s ok. Revisit this discussion at next appointment or when rapport is built.
- Greater than AIDS PrEP campaign “Powered by PrEP”: POC centered messaging including testimonials in English and Spanish
- REACH LA’s WAP campaign
- Black AIDS Institute: black cis women
- Cardea: HIV Care for Native patients
- Labeling transwomen as MSM
- MSM of color - Leo Moore presentation
- People of trans experience - evidence of lack of interaction with gender-affirming hormones
- Reproductive health - evidence of safety in conceiving, pregnancy & lactation. (HIVE toolkit)
- Seicus PrEP Education for Youth-Serving Primary Care Providers Toolkit
- Lack of diversity in healthcare Infographic
- National LGBT Health Education Center: “Understanding and Addressing the Social Determinants of Health for Black LGBTQ People”
- Same-day Oral PrEP quick guide: https://www.ebgtz.org/resource/same-day-prep/
PrEP Background
 We will begin reviewing PrEP by introducing the different medications that are available and covering their effectiveness. Echoing some of the trends that were mentioned under HIV epidemiology, this section will also work to highlight the inequities in PrEP delivery and how we can work to improve them.
We will begin reviewing PrEP by introducing the different medications that are available and covering their effectiveness. Echoing some of the trends that were mentioned under HIV epidemiology, this section will also work to highlight the inequities in PrEP delivery and how we can work to improve them.
Medications Available
PrEP encompasses a group of medications meant to be taken by someone who is HIV negative to prevent acquiring an HIV infection.
PRE = Before
EXPOSURE = Coming into potential contact with HIV
PROPHYLAXIS = Treatment to prevent an infection
There are three different medications available for PrEP. Brand names are provided in bold.
- Emtricitabine 200mg & tenofovir disoproxil fumarate 300mg (F/TDF). Truvada
- - Oral tablet approved for PrEP use in July 2012
- Emtricitabine 200mg & tenofovir alafenamide 25mg (F/TAF). Descovy
- - Oral tablet approved for PrEP use in October 2019
- Cabotegravir 600mg in 3mL (CAB). Apretude
- - Intramuscular injection approved for PrEP use in December 2021
- - Cabotegravir 30mg is also available in an oral form called Vocabria. There is an optional oral lead-in for 30 days before starting the injectable form.
Effectiveness
The majority of existing PrEP studies have focused on the use of oral F/TDF (Truvada) to prevent sexual transmission of HIV in both assigned sexes (AMAB & AFAB), regardless of sexual orientation. (9)
Grant, Anderson, P. L., McMahan, V., Liu, A., Amico, K. R., Mehrotra, M., Hosek, S., Mosquera, C., Casapia, M., Montoya, O., Buchbinder, S., Veloso, V. G., Mayer, K., Chariyalertsak, S., Bekker, L.-G., Kallas, E. G., Schechter, M., Guanira, J., Bushman, L., … Glidden, D. V. (2014). Uptake of pre-exposure prophylaxis, sexual practices, and HIV incidence in men and transgender women who have sex with men: a cohort study. The Lancet Infectious Diseases, 14(9), 820–829
https://doi.org/10.1016/S1473-3099(14)70847-3
Grant, Lama, J. R., Anderson, P. L., McMahan, V., Liu, A. Y., Vargas, L., Goicochea, P., Casapía, M., Guanira-Carranza, J. V., Ramirez-Cardich, M. E., Montoya-Herrera, O., Fernández, T., Veloso, V. G., Buchbinder, S. P., Chariyalertsak, S., Schechter, M., Bekker, L.-G., Mayer, K. H., Kallás, E. G., … Glidden, D. V. (2010). Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. The New England Journal of Medicine, 363(27), 2587–2599.
https://doi.org/10.1056/NEJMoa1011205
Baeten, Donnell, D., Mugo, N. R., Ndase, P., Thomas, K. K., Campbell, J. D., Wangisi, J., Tappero, J. W., Bukusi, E. A., Cohen, C. R., Katabira, E., Ronald, A., Tumwesigye, E., Were, E., Fife, K. H., Kiarie, J., Farquhar, C., John-Stewart, G., Kidoguchi, L., … Celum, C. (2014). Single-agent tenofovir versus combination emtricitabine plus tenofovir for pre-exposure prophylaxis for HIV-1 acquisition: an update of data from a randomised, double-blind, phase 3 trial. The Lancet Infectious Diseases, 14(11), 1055–1064.
https://doi.org/10.1016/S1473-3099(14)70937-5
Thigpen, Kebaabetswe, P. M., Paxton, L. A., Smith, D. K., Rose, C. E., Segolodi, T. M., Henderson, F. L., Pathak, S. R., Soud, F. A., Chillag, K. L., Mutanhaurwa, R., Chirwa, L. I., Kasonde, M., Abebe, D., Buliva, E., Gvetadze, R. J., Johnson, S., Sukalac, T., Thomas, V. T., … Brooks, J. T. (2012). Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. The New England Journal of Medicine, 367(5), 423–434
https://doi.org/10.1056
To date, only one study has specifically investigated the efficacy of tenofovir disoproxil fumarate among people who inject drugs (PWIDs). (10) Like all medications, it was determined early on that the effectiveness of PrEP varies greatly with adherence.
Choopanya, Martin, M., Suntharasamai, P., Sangkum, U., Mock, P. A., Leethochawalit, M., Chiamwongpaet, S., Kitisin, P., Natrujirote, P., Kittimunkong, S., Chuachoowong, R., Gvetadze, R. J., McNicholl, J. M., Paxton, L. A., Curlin, M. E., Hendrix, C. W., & Vanichseni, S. (2013). Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir study): a randomised, double-blind, placebo-controlled phase 3 trial. The Lancet (British Edition), 381(9883), 2083–2090
https://doi.org/10.1016/S0140-6736(13)61127-7
Martin, Vanichseni, S., Suntharasamai, P., Sangkum, U., Mock, P. A., Chaipung, B., Worrajittanon, D., Leethochawalit, M., Chiamwongpaet, S., Kittimunkong, S., Gvetadze, R. J., McNicholl, J. M., Paxton, L. A., Curlin, M. E., Holtz, T. H., Samandari, T., & Choopanya, K. (2016). Factors associated with the uptake of and adherence to HIV pre-exposure prophylaxis in people who have injected drugs: an observational, open-label extension of the Bangkok Tenofovir study. The Lancet HIV, 4(2), e59–e66.
https://doi.org/10.1016/S2352-3018(16)30207-7
These initial landmark trials established that with daily adherence…
F/TDF (Truvada) reduces the risk of acquiring HIV through sex by 99% and through IVDU by 74%.
The DISCOVER trial compared F/TDF (Truvada) directly to F/TAF (Descovy). This study only included MSM/TGWSM. It was concluded that F/TAF (Descovy) is non-inferior to F/TDF (Truvada) in preventing sexual transmission among this select patient population. (11)
Mayer, Molina, J.-M., Thompson, M. A., Anderson, P. L., Mounzer, K. C., De Wet, J. J., DeJesus, E., Jessen, H., Grant, R. M., Ruane, P. J., Wong, P., Ebrahimi, R., Zhong, L., Mathias, A., Callebaut, C., Collins, S. E., Das, M., McCallister, S., Brainard, D. M., … Hare, C. B. (2020). Emtricitabine and tenofovir alafenamide vs emtricitabine and tenofovir disoproxil fumarate for HIV pre-exposure prophylaxis (DISCOVER): primary results from a randomised, double-blind, multicentre, active-controlled, phase 3, non-inferiority trial. The Lancet (British Edition), 396(10246), 239–254.
https://doi.org/10.1016/S0140-6736(20)31065-5
The HIV Prevention Trials Network (HPTN) 083 and 084 were then performed in similar fashion and concluded that cabotegravir (Apretude) is superior to F/TDF (Truvada) in MSM/TGWSM and cisgender women, respectively. (12)
Landovitz, Donnell, D., Clement, M. E., Hanscom, B., Cottle, L., Coelho, L., Cabello, R., Chariyalertsak, S., Dunne, E. F., Frank, I., Gallardo-Cartagena, J. A., Gaur, A. H., Gonzales, P., Tran, H. V., Hinojosa, J. C., Kallas, E. G., Kelley, C. F., Losso, M. H., Madruga, J. V., … Sued, O. (2021). Cabotegravir for HIV prevention in cisgender men and transgender women. The New England Journal of Medicine, 385(7), 595–608.
https://doi.org/10.1056/NEJMoa2101016
Delany-Moretlwe, Hughes, J. P., Bock, P., Hunidzarira, P., Kalonji, D., Kayange, N., Makhema, J., Mandima, P., Mathew, C., Spooner, E., Mpendo, J., Mukwekwerere, P., Mgodi, N., Ntege, P. N., Nakabiito, C., Nuwagaba-Biribonwoha, H., Panchia, R., Singh, N., Farrior, J., … Marzinke, M. A. (2022). Cabotegravir for the prevention of HIV-1 in women: results from HPTN 084, a phase 3, randomised clinical trial. The Lancet (British Edition), 399(10337), 1779–1789.
https://doi.org/10.1016/S0140-6736(22)00538-4
It is worth mentioning that the superiority of cabotegravir is primarily derived from improved patient adherence as it does not rely on the need for daily ingestion. Subset analysis does show that effectiveness was similar when F/TDF (Truvada) levels indicated proper adherence. Biochemically, they are essentially equivalent.
There has also been mounting interest and investigation of event-driven PrEP (ED-PrEP), also known as on-demand, non-daily, intermittent, or 2-1-1 PrEP. Rather than daily ingestion, ED-PrEP is a method where PrEP is taken shortly before sex and then the following two days.
ED-PrEP has only been studied using F/TDF (Truvada) to prevent the sexual acquisition of HIV among men who have sex with men (MSM). Existing studies have found that ED-PrEP is non-inferior to daily intake. (13) This type of use is not currently FDA approved in the U.S. though according to the latest CDC Guidelines, it may be considered in select patients. (14)
Centers for Disease Control and Prevention. (2021). Preexposure prophylaxis for the prevention of HIV infection in the United States – 2021 update a clinical practice guideline. U.S. Department of Health and Human Services.
https://www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2021.pdf
Molina, Capitant, C., Spire, B., Pialoux, G., Cotte, L., Charreau, I., Tremblay, C., Le Gall, J.-M., Cua, E., Pasquet, A., Raffi, F., Pintado, C., Chidiac, C., Chas, J., Charbonneau, P., Delaugerre, C., Suzan-Monti, M., Loze, B., Fonsart, J., … Delfraissy, J.-F. (2015). On-demand preexposure prophylaxis in men at high risk for HIV-1 infection. The New England Journal of Medicine, 373(23), 2237–2246.
https://doi.org/10.1056/NEJMoa1506273
Antoni, Tremblay, C., Delaugerre, C., Charreau, I., Cua, E., Rojas Castro, D., Raffi, F., Chas, J., Huleux, T., Spire, B., Capitant, C., Cotte, L., Meyer, L., Molina, J.-M., Aboulker, J.-P., Bajos, N., Baril, J.-G., Besnier, M., Binesse, J., … Chidiac, C. (2020). On-demand pre-exposure prophylaxis with tenofovir disoproxil fumarate plus emtricitabine among men who have sex with men with less frequent sexual intercourse: a post-hoc analysis of the ANRS IPERGAY trial. The Lancet HIV, 7(2), e113–e120.
https://doi.org/10.1016/S2352-3018(19)30341-8
Take Home Points
There are many nuances to what was just discussed, which will be further detailed in the coming sections. For now, the key take-home point is that ALL forms of PrEP are highly effective in preventing HIV infections and should, at the very least, be mentioned to your patients.
Important PrEP Epidemiology
As alluded to in the initial section on HIV epidemiology, reductions in HIV incidence have not been favorable among the most critical patient populations.
Black and Latino patients make up the lionshare of new HIV cases every year yet, account for a small proportion of PrEP use (Figure 11).
Females account for nearly a fifth of new HIV infections but make up a meager 8% of all PrEP prescriptions (Figure 12).
By region, the South accounts for the majority of new HIV infections every year but PrEP use is limited to 39% of all active prescriptions (Figure 13).
Taken altogether, we now understand that PrEP is highly efficacious though its impact on public health measures has been rather restricted. It is no coincidence that the inequalities we see in PrEP care reflect the same patterns we see in so many other aspects of society.
Now, more than ever before, we must work together to improve these injustices to allow the potential of PrEP to take hold. Whether you are a general provider with no prior experience or a seasoned veteran in infectious diseases, the following sections will work to improve your comfort and delivery of PrEP care, especially to those who need it most.
Click image for full size view
Click the title of the figure to view source.
Figure 11 - Percentage of PrEP use in 2021 and HIV incidence in 2020 by Race
Source: AIDSVu. (n.d.). Deeper look: PrEP
https://aidsvu.org/resources/deeper-look-prep/
Figure 12 - Percentage of PrEP use by sex, 2021.
Source: AIDSVu. (n.d.). Deeper look: PrEP
https://aidsvu.org/resources/deeper-look-prep/
Click for full size view
Figure 13 - PrEP Use in the Southern Region of the U.S., 2021.
Source: AIDSVu. (n.d.). Deeper look: PrEP
https://aidsvu.org/resources/deeper-look-prep/
Patient Eligibility
 This section will cover who is eligible for PrEP by reviewing the indications and contraindications. There are also several situations and important considerations that must be discussed in order to safely provide PrEP. Although this section will introduce some technical topics that can create further confusion, do not be deterred as they occur infrequently and useful resources exist to help guide you through them.
This section will cover who is eligible for PrEP by reviewing the indications and contraindications. There are also several situations and important considerations that must be discussed in order to safely provide PrEP. Although this section will introduce some technical topics that can create further confusion, do not be deterred as they occur infrequently and useful resources exist to help guide you through them.
Indications
The CDC published the latest national guideline for PrEP in December 2021. It includes several tables that detail all of the patient indications for PrEP use which can be found here.
The following table summarizes this information with minor modifications to improve simplicity and includes certain details that were omitted.
| Indications for Pre-Exposure Prophylaxis |
|---|
|
PrEP is indicated if ALL of the following applies:
|
|
Risk Factors:
|
|
Indications for Specific Therapies:
|
|
Footnotes
Eisinger RW, Dieffenbach CW, Fauci AS. HIV Viral Load and Transmissibility of HIV Infection: Undetectable Equals Untransmittable. JAMA. 2019;321:451-2. Source: Centers for Disease Control and Prevention. (2022). Diagnoses of HIV infection in the United States and dependent areas, 2020. U.S. Department of Health and Human Services. Todd J, Grosskurth H, Changalucha J, Obasi A, Mosha F, Balira R, Orroth K, Hugonnet S, Pujades M, Ross D, Gavyole A, Mabey D, Hayes R. Risk factors influencing HIV infection incidence in a rural African population: a nested case‐control study.. J Infect Dis 2006;193(3):458‐466. Wald A, Link K. Risk of Human Immunodeficiency Virus infection in herpes simplex virus type 2 ‐ seropositive persons: a meta‐analysis. The Journal of Infectious Diseases 2002;185:45‐52. Freeman EE, Weiss HA, Glynn JR, Cross PL, Whitworth JA, Hayes RJ. Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta‐analysis of longitudinal studies. AIDS 2006;20(1):73‐83. |
| Table 1. Indications to utilize PrEP |
Contraindications
| Contraindications for Pre-Exposure Prophylaxis |
|---|
|
PrEP is not indicated if ANY of the following applies:
|
|
Footnotes
|
| Table 2. Contraindications to utilize PrEP |
Careful Considerations
- Non-occupational postexposure prophylaxis (nPEP)
- Suspicion for HIV Acquisition During the Inter-test Reactivity Interval (ITRI)
- Delayed Seroconversion
- Other Considerations
Non-occupational postexposure prophylaxis (nPEP)
Postexposure prophylaxis (PEP) refers to the use of antiretroviral medications (ARVs) to prevent HIV acquisition when there was potential exposure in the past 72 hours.
There are two forms of PEP based on the setting where the exposure event occurred. It is termed occupational PEP (oPEP) if the exposure occurred among healthcare personnel in a healthcare setting and non-occupational PEP (nPEP) for all other scenarios.
The following algorithm adopted from the National HIV Curriculum website was created using the 2016 CDC guidelines for nPEP (Figure 14). (19)
Centers for Disease Control and Prevention. (2018). Updated guidelines for antiretroviral postexposure prophylaxis after sexual, injection drug use, or other nonoccupational exposure to HIV—United States, 2016. U.S. Department of Health and Human Services.
https://www.cdc.gov/hiv/pdf/programresources/cdc-hiv-npep-guidelines.pdf
The first determination that needs to be made is the level of risk for HIV acquisition. Substantial risk is defined as direct exposure of mucosal sites or the bloodstream (non-intact skin or percutaneous) with specific bodily fluids (blood, genital fluids, or breastmilk) when the source patient is HIV+.
This is a good start though it requires you to know the HIV-status of the source patient, a detail typically unknown in cases that occur outside of medical settings. It also disregards the latest evidence of U=U. (15) The risk may be negligible if the event was sexual contact with an HIV+ source who has had undetectable viral levels for more than six consecutive months.
Eisinger, Dieffenbach, C. W., & Fauci, A. S. (2019). HIV viral load and transmissibility of HIV infection: undetectable equals untransmittable. JAMA: The Journal of the American Medical Association, 321(5), 451–452.
https://doi.org/10.1001/jama.2018.21167
Click image for full size view
Click the title of the figure to view source.
Figure 14 - Algorithm for use of non-occupational post-exposure prophylaxis (nPEP)
Source: Centers for Disease Control and Prevention, U.S. Department of Health and Human Services. Updated Guidelines for Antiretroviral Postexposure Prophylaxis After Sexual, Injection Drug Use, and Other Nonoccupational Exposure to HIV – United States, 2016.
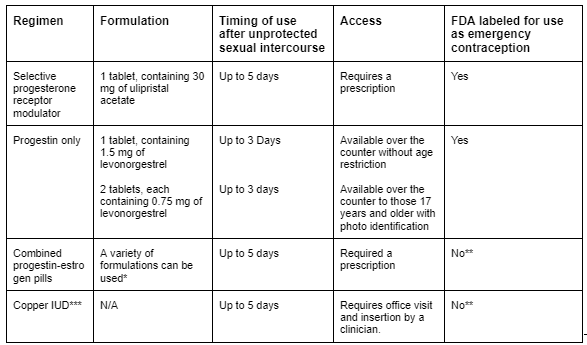
Table 3 - Available Methods of Emergency Contraception
Source: Emergency contraception. Practice Bulletin No. 152. American College of Obstetricians and Gynecologists. Obstet Gynecol 2015;126:e1–11.
In most cases, the substantial risk is hard to determine; thus, we must rely on our knowledge of HIV transmission probability (Figure 1) and other related factors (risk calculator).
Patients placed on nPEP should be asked if they would like to transition to PrEP after completion of the 28-day course. HIV-RNA and HIV-Ag/Ab should be collected during the last week of nPEP, and if they are both negative, patients can be switched to PrEP.
This can be a daunting and confusing “gray” area, which is why the National Clinician Consultation Center also has a dedicated hotline for nPEP questions.
(888) 448-4911 9am-8pm. Eastern Time Monday – Friday,
11am – 8pm. Eastern Time on weekends & holidays.
For further information, refer to the National HIV Curriculum website and the AETC’s nPEP toolkit.
One final note. The timing of nPEP roughly coincides with the timing of emergency contraception (EC). This critical component of care should be incorporated into the discussion when applicable. ACOG Guidelines on EC.
Suspicion for HIV Acquisition During the Inter-test Reactivity Interval (ITRI)
As previously discussed, the early stages of HIV infection pose many challenges for our laboratory and clinical approaches. In particular, the ITRI, which is the narrow time period when HIV-RNA is positive, but the HIV-Ag/Ab test is negative.
Current guidelines suggest...
PrEP should be deferred until HIV-RNA test results are obtained if, in the past four weeks, the patient had potential exposure to HIV AND experienced symptoms of the acute retroviral syndrome (ARS). (20)
Centers for Disease Control and Prevention. (2021). Preexposure prophylaxis for the prevention of HIV infection in the United States – 2021 update a clinical practice guideline. U.S. Department of Health and Human Services.
https://www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2021.pdf
Now, there are some underlying subtleties to this approach. First, as we now know, “potential exposures” can range widely in their risk of HIV transmission. For example, penile-vaginal sex with a condom poses a much lower risk than unprotected receptive anal intercourse.
For PrEP, there is no consensus as to where the threshold falls for what is considered a substantial risk. However, some parallels can be drawn to nPEP.
Second, given that ARS symptoms are inconsistent and obscure, we must consider the likelihood of other conditions. Perhaps, the patient with fever and fatigue was recently diagnosed with COVID-19. Delaying PrEP initiation would not be appropriate in this setting. Similar to the Well’s criteria for DVT and PE that asks if an alternative diagnosis is more likely, we suggest using other contextual factors to help make this decision.
A slight revision using these points of clarification would be…
PrEP should be deferred until HIV-RNA test results are obtained if, in the past four weeks, the patient had potential exposure to HIV with substantial risk AND experienced symptoms of the acute retroviral syndrome (ARS), unexplained by a more probable cause.
If the patient meets this suggested criteria, HIV-RNA and HIV-Ab/Ag should be collected and results reviewed prior to starting PrEP.
Again, utilize the National Clinicians Consultation Center hotline if you are ever in need of support.
(888) 448-4911 9am-8pm. Eastern Time Monday – Friday,
11am – 8pm. Eastern Time on weekends & holidays.
For further information, refer to the National HIV Curriculum website and the AETC’s nPEP toolkit.
Delayed Seroconversion
All of the medications in all three formulations of PrEP have activity against the HIV virus. They each require a certain level of concentration to ensure adequate protection. Since PrEP is a medication that relies heavily on patient adherence, there have been a few documented HIV infections that occurred during PrEP use. When drug levels fall below this critical threshold, HIV can permanently establish itself as a new infection. The residual amounts of PrEP medication that were inadequate in preventing infection go on to suppress the virus partially.
This partial suppression can sometimes delay the body’s natural immune response of creating antibodies. This phenomenon, called delayed seroconversion, can prolong the window period of HIV-Ag/Ab tests and thus, the ITRI.
The average time of delayed seroconversion for oral PrEP (F/TDF and F/TAF) is about 30 days (range 7-68 days) and for injectable PrEP (CAB), 80 days (range 35-185). (21) Because of this, there are minor changes for the lab recommendations when a patient was recently on PrEP.
Marzinke, Grinsztejn, B., Fogel, J. M., Piwowar-Manning, E., Li, M., Weng, L., McCauley, M., Cummings, V., Ahmed, S., Haines, C. D., Bushman, L. R., Petropoulos, C., Persaud, D., Adeyeye, A., Kofron, R., Rinehart, A., St Clair, M., Rooney, J. F., Pryluka, D., … Eshleman, S. H. (2021). Characterization of Human Immunodeficiency Virus (HIV) Infection in Cisgender Men and Transgender Women Who Have Sex With Men Receiving Injectable Cabotegravir for HIV Prevention: HPTN 083. The Journal of Infectious Diseases, 224(9), 1581–1592.
https://doi.org/10.1093/infdis/jiab152
Other Considerations
Although they are not strong contraindications, the following details warrant careful consideration before initiating PrEP. Please do not let the details of this section deter you from discussing and providing PrEP with your patients. These intricate aspects of PrEP are included to fine-tune your understanding and address some common questions that may arise.
| Pregnancy and breastfeeding |
There is an increased risk of acquiring HIV during periods of conception, pregnancy, and breastfeeding. (22). F/TDF (Truvada) is the recommended agent for this patient population; it does not cause any adverse pregnancy outcomes. The effects on infants exposed to F/TDF during lactation have not been well studied though existing studies suggest very limited drug exposure. (23) Mugwanya, John-Stewart, G., & Baeten, J. (2017). Safety of oral tenofovir disoproxil fumarate-based HIV pre-exposure prophylaxis use in lactating HIV-uninfected women. Expert Opinion on Drug Safety, 16(7), 867–871. Benaboud, Pruvost, A., Coffie, P. A., Ekouévi, D. K., Urien, S., Arrivé, E., Blanche, S., Théodoro, F., Avit, D., Dabis, F., Tréluyer, J.-M., & Hirt, D. (2011). Concentrations of tenofovir and emtricitabine in breast milk of HIV-1-infected women in Abidjan, Côte d’Ivoire, in the ANRS 12109 TEmAA study, step 2. Antimicrobial Agents and Chemotherapy, 55(3), 1315–1317. Waitt, Olagunju, A., Nakalema, S., Kyohaire, I., Owen, A., Lamorde, M., & Khoo, S. (2018). Plasma and breast milk pharmacokinetics of emtricitabine, tenofovir and lamivudine using dried blood and breast milk spots in nursing African mother-infant pairs. Journal of Antimicrobial Chemotherapy, 73(4), 1013–1019. Mugo, Heffron, R., Donnell, D., Wald, A., Were, E. O., Rees, H., Celum, C., Kiarie, J. N., Cohen, C. R., Kayintekore, K., & Baeten, J. M. (2011). Increased risk of HIV-1 transmission in pregnancy: a prospective study among African HIV-1-serodiscordant couples. AIDS (London), 25(15), 1887–1895. Thomson, Hughes, J., Baeten, J. M., John-Stewart, G., Celum, C., Cohen, C. R., Ngure, K., Kiarie, J., Mugo, N., & Heffron, R. (2018). Increased risk of HIV acquisition among women throughout pregnancy and during the postpartum period: a prospective per-coital-act analysis among women with HIV-infected partners. The Journal of Infectious Diseases, 218(1), 16–25 Further investigation is needed for F/TAF (Descovy) and CAB (Apretude) in this patient population. Existing data among HIV+ women taking these agents for HIV treatment does show favorable safety profiles. Use of these agents as PrEP may be considered if there is a shared and informed decision that the risks outweigh the benefits. Information should be anonymously submitted if any form of PrEP is used during pregnancy at http://www.apregistry.com/ |
| Disorders that affect bone mineral density (BMD) |
Both F/TDF (Truvada) and F/TAF are known to lower BMD. Although F/TDF (Truvada) is often cited as the more “toxic” agent on bone health when compared to F/TAF (Descovy), all existing studies have found only small and clinically insignificant impacts on BMD. Furthermore, the minor losses in BMD caused by F/TDF (Truvada) were fully recovered within 12-18 months of drug discontinuation. (24) Jones, Restrepo, D., Kasowitz, A., Korenstein, D., Wallenstein, S., Schneider, A., & Keller, M. J. (2007). Risk factors for decreased bone density and effects of HIV on bone in the elderly. Osteoporosis International, 19(7), 913–918. Cazanave, Dupon, M., Lavignolle-Aurillac, V., Barthe, N., Lawson-Ayayi, S., Mehsen, N., Mercié, P., Morlat, P., Thiébaut, R., & Dabis, F. (2008). Reduced bone mineral density in HIV-infected patients: prevalence and associated factors. AIDS (London), 22(3), 395–402. Brown, & Qaqish, R. B. (2006). Antiretroviral therapy and the prevalence of osteopenia and osteoporosis: a meta-analytic review. AIDS (London), 20(17), 2165–2174. Nevertheless, preference towards F/TAF (Descovy) or CAB (Apretude) may be warranted in those with prior pathologic fracture and/or pre-existing conditions related to poor bone health such as: osteopenia/osteoporosis, type I diabetes, celiac disease (or other malabsorptive conditions), hyperthyroidism, hyperparathyroidism, osteogenesis imperfecta, multiple myeloma, chronic use of glucocorticosteroids or hormone blockers. None of these conditions are hard contraindications to PrEP use and it should be discussed that BMD declines are much more significant and irreversible once HIV is contracted. |
| Drugs with potentially concerning interactions |
Aside from the previously mentioned drugs that are absolutely contraindicated, other drugs may cause interactions that warrant careful monitoring for toxicities. Especially those that are nephrotoxic. Utilize https://hiv-druginteractions.org/checker to make informed decisions for your patients. |
| Hepatitis B virus (HBV) infection |
The medications in oral formulations of PrEP (F/TDF and F/TAF) also have activity against HBV. Among patients with a positive hepatitis B surface antigen (HBsAg), abrupt discontinuation of oral PrEP can potentially cause hepatic flares. More recent analyses have gone on to say that HBsAg+ individuals with normal or near normal transaminase levels have an extremely low risk of hepatic flare when stopping PrEP. The CDC guidelines specifically states that PrEP should not be withheld while waiting for HBV results. (25) Nevertheless, the potential of causing hepatic flares is not to be taken lightly and due to this concept, ED-PrEP is not recommended for patients who are HBsAg+. |
| Patients ≥ 65 years old |
No direct studies have been performed on this age group. Kidney function and bone mineral density naturally deteriorates with age so caution should be used with select PrEP agents. |
| Table 5. Careful considerations regarding PrEP Use | |
PrEP Initiation
 Once you and the patient have agreed to start PrEP, there are important aspects of counseling and management that needs to be done. This section will review these topics to help you provide comprehensive care that extends a bit beyond HIV prevention. Keep in mind that these topics are meant to be addressed across multiple visits as you build longitudinal care with your patients.
Once you and the patient have agreed to start PrEP, there are important aspects of counseling and management that needs to be done. This section will review these topics to help you provide comprehensive care that extends a bit beyond HIV prevention. Keep in mind that these topics are meant to be addressed across multiple visits as you build longitudinal care with your patients.
Counseling on Use
Building a good understanding of the underlying social context is a fundamental prerequisite to delivering effective and compassionate PrEP care.
Counseling on PrEP is naturally a sensitive topic as it broaches personal information and socially controversial issues. There are many ways to medically discuss the components of care with a patient. Similar to the subtleties mentioned in the section on how to take a sexual and substance use history, the counseling involved during PrEP initiation requires a mindful approach. Minor adjustments in our language may be critical in establishing a good rapport and ensuring proper patient understanding and follow-up care.
The inequities seen in PrEP make this point particularly important. The negative stigma that continues to be associated with HIV and issues related to medical mistrust are just some of the many reasons why PrEP is not being adequately delivered to those who need it most.
The style and approach in which you discuss PrEP is an ever-evolving process that will require trial and error. Continue refining your approach while including the following counseling points during your initial discussions of PrEP.
| PrEP Initiation Patient Counseling Checklist |
|---|
|
I have discussed ALL of the following points with the patient |
|
| Table 6. PrEP Initiation Patient Counseling Checklist |
Intake Laboratory Testing
The 2021 PrEP Guidelines published by the CDC include updated tables that organize the recommended laboratory tests for oral and intramuscular PrEP. As shown in Table 5. Careful considerations regarding PrEP Use and Table 7. Recommended Labs for Pre-exposure Prophylaxis Using F_TDF or F_TAF
While these tables present comprehensive information that highlight the subtleties of PrEP management, they can be challenging to interpret, especially for clinicians newly introduced to PrEP.
Below are modified tables for PrEP labs that work to simplify the guidelines. These suggestions are all drawn from related evidence-based studies. They aim to enhance patient care without sacrificing patient safety.
Note that HIV-RNA at initiation is recommended in select scenarios out of concern for delayed seroconversion or HIV acquisition during the ITRI as previously discussed. Also, labs recommended at discontinuation are represented in bold.
Detailed explanation of these lab recommendations are found in the Explanation of Suggesting Labs section.
- Recommended Labs for PrEP Using F/TDF or F/TAF
- Recommended Labs for PrEP Using Injectable Cabotegravir
| Recommended Labs for PrEP Using F/TDF or F/TAF | ||||
|---|---|---|---|---|
| Test | Initiation | Q3 | Q6 | As Indicated |
| HIV-Ab/Ag | ● | ● | ||
| HIV-RNA |
If ANY of the following:
|
|
||
| Complete Metabolic Panel | ● | ● |
|
|
| STI Panel2 | ● | ● |
|
|
| Pregnancy Test | ● | ● |
|
|
| Hepatitis A Ab Total | ● | |||
| Hepatitis B Panel3 | ● |
|
||
| Hepatitis C Ab4 | ● |
|
||
|
Footnotes
|
||||
| Table 7. Recommended labs for PrEP Using F/TDF or F/TAF | ||||
| Recommended Labs for PrEP Using Injectable Cabotegravir | ||||
|---|---|---|---|---|
| Test | Initiation | At 1mo f/u | Q2 Months | As Indicated |
| HIV-RNA | ● | ● | ● |
|
| Complete Metabolic Panel | ● |
|
||
| STI Panel1 | ● |
|
||
| Pregnancy Test | ● |
|
||
| Hepatitis A Ab | ● | |||
| Hepatitis B Panel2 | ● |
|
||
| Hepatitis C Ab3 | ● |
|
||
|
Footnotes
|
||||
| Table 8. Recommended Labs for PrEP Using Injectable Cabotegravir | ||||
Primary Care Considerations
General health screening
Sometimes, patients engaged in PrEP care do not routinely see other providers to address other aspects of preventive medicine. PrEP follow-up visits are generally straightforward and can be quick visits. Utilize this opportunity to incorporate these Grade A and B United States Preventive Services Task Force (USPSTF) recommendations. (29)
U.S. Preventive Services Task Force. A & B Recommendations.
https://www.uspreventiveservicestaskforce.org/uspstf/recommendation-topics/uspstf-a-and-b-recommendations
| Recommendation | Indication | Test (most commonly used) |
|---|---|---|
| Cervical Cancer Screening | Women 21-65yo | Pap-smear (w/ or w/ out HPV) |
| Colorectal Cancer Screening | Adults 45-75yo | FIT, colonoscopy |
| Intimate Partner Violence | Women of reproductive age | PVS, AAS |
| Latent Tuberculosis | Adults with a risk factor: born or lived in countries with high TB prevalence, lived or is living in homeless shelters or correctional facilities, currently homeless, etc. | QuantiFERON-TB Gold, TST (PPD) |
| Hypertension | All adults | Blood pressure |
| Diabetes | Adults 35-70yo with BMI>25 | HbA1c |
| Depression | Everyone | PHQ-2/ PHQ-9 EPDS in postpartum and pregnant women |
| Folic Acid to Prevent Neural Tube Defects | All women planning or capable of pregnancy | Part of Sexual History (6 P’s) |
| Primary Prevention of Cardiovascular Disease With Statin Medication | Adults 40-75yo | ASCVD Risk Calculator |
| Tobacco Smoking Screening and Cessation Counseling | All adults | Directly Asking and Counseling |
| Alcohol Abuse Screening | All adults | AUDIT-C |
| Drug Abuse Screening | All adults | NIDA Quick Screen-C |
| Table 9. Relevant Screening Recommendations | ||
Vaccinations
For the purposes of PrEP, the following vaccinations are most relevant.
Source: https://www.cdc.gov/vaccines/schedules/hcp/imz/adult.html#note-mmr
| Vaccine | Indication |
|---|---|
| COVID-19 | Primary series and boosters recommended for everyone. |
| Hepatitis A | No immunity based on labs. |
| Hepatitis B | No immunity based on labs (HBsAb<10mIU/mL) |
| Human Papillomavirus | Recommended up to 26yo. If not previously vaccinated, shared decision with patients between 27-45yo |
| Hypertension | All adults |
| Influenza | Recommended for everyone, annually. |
| Meningococcal B | Shared decision for patients 16-23yo |
| Monkeypox
(30)
Centers for Disease Control and Prevention. (2022). Monkeypox vaccination basics. Department of Health and Human Services. |
Rapidly changing guidelines based on local health departments though for currently: adults with risk factors such as MSM/TGWSM, engages in transactional sex, or had a known exposure. |
| Pneumococcal | Adults 19-64yo with a risk factor: alcoholism, cigarette smoker, DM, etc. |
| Tetanus, diphtheria, and pertussis (Tdap) | Booster (Td or Tdap) generally recommended every 10 years |
| Zoster | All adults over 50yo |
| Table 10. Relevant Vaccination Recommendations | |
Family Planning
 Given that PrEP is most often discussed along with sexual practices, it is good practice to incorporate components of family planning into your approach. There continue to be high rates of unintended pregnancies, particularly among the same groups disproportionately affected by HIV.
(31) At a time when women’s reproductive rights are constantly being challenged, it is increasingly important to provide guidance, support, and comfort to women of childbearing potential. Utilize "the 6 P’s” framework to expand on topics related to family planning and reproductive health.
Given that PrEP is most often discussed along with sexual practices, it is good practice to incorporate components of family planning into your approach. There continue to be high rates of unintended pregnancies, particularly among the same groups disproportionately affected by HIV.
(31) At a time when women’s reproductive rights are constantly being challenged, it is increasingly important to provide guidance, support, and comfort to women of childbearing potential. Utilize "the 6 P’s” framework to expand on topics related to family planning and reproductive health.
Finer, & Zolna, M. R. (2016). Declines in Unintended Pregnancy in the United States, 2008–2011. The New England Journal of Medicine, 374(9), 843–852.
https://doi.org/10.1056/NEJMsa1506575
Coding for PrEP Visits
Below are the recommended visit codes for PrEP management.
This may vary based on your institution.

- Z20.6Z20.6 (Contact with and (suspected) exposure to HIV)
- Z79.899 (Other long term drug therapy) If PrEP is started or continued
and at least one of the following:
- Z72.5 (high risk sexual behavior) if there are sexual risk factors
- W46.1XXD (contact with contaminated hypodermic needle) if there are IVDU risk factors
Billing:
- If PrEP is the only topic addressed
- 99401 approximately 15 minutes
- 99402 approximately 30 minutes
- 99403 approximately 45 minutes
- 99404 approximately 60 minutes
- If multiple health topics are addressed, use normal billing codes based on complexity (e.g. 99213, 99214)
PrEP Continuation Visit
 Follow up visits are important opportunities to ensure
that the benefits of PrEP are maximized and the risks are mitigated. This section covers important counseling topics for both continuing and discontinuing PrEP. During these continuation visits, previously mentioned aspects of care should slowly be
offered and provided as well.
Follow up visits are important opportunities to ensure
that the benefits of PrEP are maximized and the risks are mitigated. This section covers important counseling topics for both continuing and discontinuing PrEP. During these continuation visits, previously mentioned aspects of care should slowly be
offered and provided as well.
Discussion points for follow up
During your follow-up PrEP visit, discuss the following topics:
| PrEP Continuation Patient Counseling Checklist |
|---|
|
| Table 11. PrEP Continuation Patient Counseling Checklist |
Addressing discontinuation
The use of PrEP should always be a shared decision with your patients. Carefully discuss the following points without placing pressure on the patient to continue.
| PrEP Continuation Patient Counseling Checklist |
|---|
|
| Table 12. PrEP Discontinuation Patient Counseling Checklist |
Advanced Topics
 This section will be covering a couple of topics that
go beyond the basics of providing PrEP such as counseling for patients who opt for event-driven PrEP and for patients who acquire HIV while on PrEP. Lastly, there will be a detailed explanation of the labs that have been suggested in prior sections.
We hope that this section works to build an even deeper understanding of PrEP.
This section will be covering a couple of topics that
go beyond the basics of providing PrEP such as counseling for patients who opt for event-driven PrEP and for patients who acquire HIV while on PrEP. Lastly, there will be a detailed explanation of the labs that have been suggested in prior sections.
We hope that this section works to build an even deeper understanding of PrEP.
Event-Driven PrEP (ED-PrEP)
Although the data is limited, event-driven PrEP (ED-PrEP) with F/TDF (Truvada) appears to be an efficacious alternative strategy, offering similar protection against HIV infections as daily dosing. ED-PrEP may be appropriate for patients who are MSM and do not prefer daily ingestion. There are a couple of important points to note and discuss with your patients:
| Event-Driven PrEP Patient Counseling Checklist |
|---|
|
|
|
| Table 13. ED-PrEP Patient Counseling Checklist Source: https://checkhimout.ca/what-is-prep/resources/ |
HIV Acquisition while on PrEP & Risk of resistance
In rare circumstances, patients may acquire an HIV infection while taking PrEP. Usually, this occurs when there are subtherapeutic drug concentrations to prevent an HIV infection, such as when there is poor adherence or acquisition during the initial week of PrEP initiation. Since these new HIV infections occur in the presence of partial drug pressure, resistance mutations may develop, which is an often-cited concern.
Although this is not a matter to be taken lightly, the resistance patterns most often caused by F/TDF (Truvada) and F/TAF (Descovy) are commonly seen and can be easily managed. (32) This is best summarized by one study that stated that we “should respect but not fear” resistance caused by oral PrEP. (33)
Parikh, & Mellors, J. W. (2016). Should we fear resistance from tenofovir/emtricitabine preexposure prophylaxis? Current Opinion in HIV & AIDS, 11(1), 49–55.
https://doi.org/10.1097/COH.0000000000000209
Tittle, Boffito, M., McOwan, A., & Whitlock, G. (2020). Antiretroviral resistance and management after pre-exposure prophylaxis. The Lancet HIV, 7(2), e84–e84.
https://doi.org/10.1016/S2352-3018(19)30404-7
Liegler, Abdel-Mohsen, M., Bentley, L. G., Atchison, R., Schmidt, T., Javier, J., Mehrotra, M., Eden, C., Glidden, D. V., McMahan, V., Anderson, P. L., Li, P., Wong, J. K., Buchbinder, S., Guanira, J. V., Grant, R. M., & iPrEx Study Team, S. T. (2014). HIV-1 drug resistance in the iPrEx preexposure prophylaxis trial. The Journal of Infectious Diseases, 210(8), 1217–1227.
https://doi.org/10.1093/infdis/jiu233
This same investigation utilized a mathematical model to predict that the number of HIV infections that can be prevented with PrEP far exceeds the number of drug-resistant infections caused by PrEP. Many analyses corroborate this. (34)
Abbas, Glaubius, R., Mubayi, A., Hood, G., & Mellors, J. W. (2013). Antiretroviral therapy and pre-exposure prophylaxis: combined impact on HIV transmission and drug resistance in South Africa. The Journal of Infectious Diseases, 208(2), 224–234.
https://doi.org/10.1093/infdis/jit150
Baggaley, Powers, K. A., & Boily, M.-C. (2011). What do mathematical models tell us about the emergence and spread of drug-resistant HIV? Current Opinion in HIV & AIDS, 6(2), 131–140.
https://doi.org/10.1097/COH.0b013e328343ad03
Gibas, van den Berg, P., Powell, V. E., & Krakower, D. S. (2019). Drug resistance during HIV pre-exposure prophylaxis. Drugs (New York, N.Y.), 79(6), 609–619.
https://doi.org/10.1007/s40265-019-01108-x
Cabotegravir is from an entirely different class of antiretroviral medication called integrase strand transfer inhibitors (INSTI). We rely heavily on INSTIs for HIV treatment and, thus, should be much more cautious. Especially since cabotegravir has a long “tail phase” where subtherapeutic drug levels allow HIV infections to take hold and develop resistance patterns. This is why current recommendations call for continued use of F/TDF (Truvada) or F/TAF (Descovy) for a year after the last cabotegravir injection. The data on this is limited and still under investigation. (35)
Landovitz, Donnell, D., Clement, M. E., Hanscom, B., Cottle, L., Coelho, L., Cabello, R., Chariyalertsak, S., Dunne, E. F., Frank, I., Gallardo-Cartagena, J. A., Gaur, A. H., Gonzales, P., Tran, H. V., Hinojosa, J. C., Kallas, E. G., Kelley, C. F., Losso, M. H., Madruga, J. V., … Sued, O. (2021). Cabotegravir for HIV prevention in cisgender men and transgender women. The New England Journal of Medicine, 385(7), 595–608.
https://doi.org/10.1056/NEJMoa2101016
Delany-Moretlwe, Hughes, J. P., Bock, P., Hunidzarira, P., Kalonji, D., Kayange, N., Makhema, J., Mandima, P., Mathew, C., Spooner, E., Mpendo, J., Mukwekwerere, P., Mgodi, N., Ntege, P. N., Nakabiito, C., Nuwagaba-Biribonwoha, H., Panchia, R., Singh, N., Farrior, J., … Marzinke, M. A. (2022). Cabotegravir for the prevention of HIV-1 in women: results from HPTN 084, a phase 3, randomised clinical trial. The Lancet (British Edition), 399(10337), 1779–1789.
https://doi.org/10.1016/S0140-6736(22)00538-4
Regardless of which PrEP medication is used, frequent testing is the most effective way to detect HIV infections as soon as possible and prevent further communal spread.
The detailed management of HIV goes beyond the purposes of this toolkit. Check with your local institution about the process of connecting patients to HIV care.
Whether or not you feel comfortable managing HIV, this is a critical time for patients to be consoled and engaged in proper care. You may be the first point of contact after a positive result.
Explanation of suggested labs
Resources
 A summary of tables, handouts, and additional information
for providers and patients.
A summary of tables, handouts, and additional information
for providers and patients.
Patient Tools
For assistance finding a PrEP Provider and paying for PrEP:
Tables
References
Centers for Disease Control and Prevention. (2021). Preexposure prophylaxis for the prevention of HIV infection in the United States – 2021 update a clinical practice guideline. U.S. Department of Health and Human Services. https://www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2021.pdf
Fiebig, Wright, D. J., Rawal, B. D., Garrett, P. E., Schumacher, R. T., Peddada, L., Heldebrant, C., Smith, R., Conrad, A., Kleinman, S. H., & Busch, M. P. (2003). Dynamics of HIV viremia and antibody seroconversion in plasma donors: implications for diagnosis and staging of primary HIV infection. AIDS (London), 17(13), 1871–1879. https://doi.org/10.1097/00002030-200309050-00005
DetermineTM HIV-1/2 Ag/Ab Combo. (n.d.). Retrieved September 19, 2022, from https://www.globalpointofcare.abbott/en/product-details/determine-1-2-ag-ab-combo.html
Branson, B.M., Owen, M.S., Wesolowshi, L.G., Bennett, B., Werner, B.G., Wroblewski, K.E., Pentella, M.A. (2014). Laboratory testing for the diagnosis of HIV infection: updated recommendations. https://doi.org/10.15620/cdc.23447
Delaney, Hanson, D. L., Masciotra, S., Ethridge, S. F., Wesolowski, L., & Owen, S. M. (2017). Time until emergence of HIV test reactivity following infection with HIV-1: implications for interpreting test results and retesting after exposure. Clinical Infectious Diseases, 64(1), 53–59. https://doi.org/10.1093/cid/ciw666
Patel, Borkowf, C. B., Brooks, J. T., Lasry, A., Lansky, A., & Mermin, J. (2014). Estimating per-act HIV transmission risk: a systematic review. AIDS (London), 28(10), 1509–1519. https://doi.org/10.1097/QAD.0000000000000298
Masciotra, Luo, W., Westheimer, E., Cohen, S. E., Gay, C. L., Hall, L., Pan, Y., Peters, P. J., & Owen, S. M. (2017). Performance evaluation of the FDA-approved DetermineTM HIV-1/2 Ag/Ab Combo assay using plasma and whole blood specimens. Journal of Clinical Virology, 91, 95–100. https://doi.org/10.1016/j.jcv.2017.03.019
Daar, Pilcher, C. D., & Hecht, F. M. (2008). Clinical presentation and diagnosis of primary HIV-1 infection. Current Opinion in HIV & AIDS, 3(1), 10–15. https://doi.org/10.1097/COH.0b013e3282f2e295
Centers for Disease Control and Prevention. (2021). Estimated HIV incidence and prevalence in the United States, 2015–2019. U.S. Department of Health and Human Services. https://www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance-supplemental-report-vol-26-1.pdf
Centers for Disease Control and Prevention. (2021). Diagnoses of HIV infection in the United States and dependent areas, 2019. U.S. Department of Health and Human Services. https://www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance-report-2018-updated-vol-32.pdf
Centers for Disease Control and Prevention. (2022). Diagnoses of HIV infection in the United States and dependent areas, 2020. U.S. Department of Health and Human Services.
https://www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance-report-2020-updated-vol-33.pdf
Kahn, & Walker, B. D. (1998). Acute human immunodeficiency virus type 1 infection. The New England Journal of Medicine, 339(1), 33–39. https://doi.org/10.1056/NEJM199807023390107
Cohen, Shaw, G. M., McMichael, A. J., & Haynes, B. F. (2011). Acute HIV-1 infection. The New England Journal of Medicine, 364(20), 1943–1954. https://doi.org/10.1056/NEJMra1011874
Mayer, Molina, J.-M., Thompson, M. A., Anderson, P. L., Mounzer, K. C., De Wet, J. J., DeJesus, E., Jessen, H., Grant, R. M., Ruane, P. J., Wong, P., Ebrahimi, R., Zhong, L., Mathias, A., Callebaut, C., Collins, S. E., Das, M., McCallister, S., Brainard, D. M., … Hare, C. B. (2020). Emtricitabine and tenofovir alafenamide vs emtricitabine and tenofovir disoproxil fumarate for HIV pre-exposure prophylaxis (DISCOVER): primary results from a randomised, double-blind, multicentre, active-controlled, phase 3, non-inferiority trial. The Lancet (British Edition), 396(10246), 239–254. https://doi.org/10.1016/S0140-6736(20)31065-5
Grant, Anderson, P. L., McMahan, V., Liu, A., Amico, K. R., Mehrotra, M., Hosek, S., Mosquera, C., Casapia, M., Montoya, O., Buchbinder, S., Veloso, V. G., Mayer, K., Chariyalertsak, S., Bekker, L.-G., Kallas, E. G., Schechter, M., Guanira, J., Bushman, L., … Glidden, D. V. (2014). Uptake of pre-exposure prophylaxis, sexual practices, and HIV incidence in men and transgender women who have sex with men: a cohort study. The Lancet Infectious Diseases, 14(9), 820–829. https://doi.org/10.1016/S1473-3099(14)70847-3
Grant, Lama, J. R., Anderson, P. L., McMahan, V., Liu, A. Y., Vargas, L., Goicochea, P., Casapía, M., Guanira-Carranza, J. V., Ramirez-Cardich, M. E., Montoya-Herrera, O., Fernández, T., Veloso, V. G., Buchbinder, S. P., Chariyalertsak, S., Schechter, M., Bekker, L.-G., Mayer, K. H., Kallás, E. G., … Glidden, D. V. (2010). Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. The New England Journal of Medicine, 363(27), 2587–2599. https://doi.org/10.1056/NEJMoa1011205
Baeten, Donnell, D., Mugo, N. R., Ndase, P., Thomas, K. K., Campbell, J. D., Wangisi, J., Tappero, J. W., Bukusi, E. A., Cohen, C. R., Katabira, E., Ronald, A., Tumwesigye, E., Were, E., Fife, K. H., Kiarie, J., Farquhar, C., John-Stewart, G., Kidoguchi, L., … Celum, C. (2014). Single-agent tenofovir versus combination emtricitabine plus tenofovir for pre-exposure prophylaxis for HIV-1 acquisition: an update of data from a randomised, double-blind, phase 3 trial. The Lancet Infectious Diseases, 14(11), 1055–1064. https://doi.org/10.1016/S1473-3099(14)70937-5
Thigpen, Kebaabetswe, P. M., Paxton, L. A., Smith, D. K., Rose, C. E., Segolodi, T. M., Henderson, F. L., Pathak, S. R., Soud, F. A., Chillag, K. L., Mutanhaurwa, R., Chirwa, L. I., Kasonde, M., Abebe, D., Buliva, E., Gvetadze, R. J., Johnson, S., Sukalac, T., Thomas, V. T., … Brooks, J. T. (2012). Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. The New England Journal of Medicine, 367(5), 423–434. https://doi.org/10.1056/NEJMoa1110711
Choopanya, Martin, M., Suntharasamai, P., Sangkum, U., Mock, P. A., Leethochawalit, M., Chiamwongpaet, S., Kitisin, P., Natrujirote, P., Kittimunkong, S., Chuachoowong, R., Gvetadze, R. J., McNicholl, J. M., Paxton, L. A., Curlin, M. E., Hendrix, C. W., & Vanichseni, S. (2013). Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir study): a randomised, double-blind, placebo-controlled phase 3 trial. The Lancet (British Edition), 381(9883), 2083–2090. https://doi.org/10.1016/S0140-6736(13)61127-7
Martin, Vanichseni, S., Suntharasamai, P., Sangkum, U., Mock, P. A., Chaipung, B., Worrajittanon, D., Leethochawalit, M., Chiamwongpaet, S., Kittimunkong, S., Gvetadze, R. J., McNicholl, J. M., Paxton, L. A., Curlin, M. E., Holtz, T. H., Samandari, T., & Choopanya, K. (2016). Factors associated with the uptake of and adherence to HIV pre-exposure prophylaxis in people who have injected drugs: an observational, open-label extension of the Bangkok Tenofovir study. The Lancet HIV, 4(2), e59–e66. https://doi.org/10.1016/S2352-3018(16)30207-7
Landovitz, Donnell, D., Clement, M. E., Hanscom, B., Cottle, L., Coelho, L., Cabello, R., Chariyalertsak, S., Dunne, E. F., Frank, I., Gallardo-Cartagena, J. A., Gaur, A. H., Gonzales, P., Tran, H. V., Hinojosa, J. C., Kallas, E. G., Kelley, C. F., Losso, M. H., Madruga, J. V., … Sued, O. (2021). Cabotegravir for HIV prevention in cisgender men and transgender women. The New England Journal of Medicine, 385(7), 595–608. https://doi.org/10.1056/NEJMoa2101016
Delany-Moretlwe, Hughes, J. P., Bock, P., Hunidzarira, P., Kalonji, D., Kayange, N., Makhema, J., Mandima, P., Mathew, C., Spooner, E., Mpendo, J., Mukwekwerere, P., Mgodi, N., Ntege, P. N., Nakabiito, C., Nuwagaba-Biribonwoha, H., Panchia, R., Singh, N., Farrior, J., … Marzinke, M. A. (2022). Cabotegravir for the prevention of HIV-1 in women: results from HPTN 084, a phase 3, randomised clinical trial. The Lancet (British Edition), 399(10337), 1779–1789. https://doi.org/10.1016/S0140-6736(22)00538-4
Molina, Capitant, C., Spire, B., Pialoux, G., Cotte, L., Charreau, I., Tremblay, C., Le Gall, J.-M., Cua, E., Pasquet, A., Raffi, F., Pintado, C., Chidiac, C., Chas, J., Charbonneau, P., Delaugerre, C., Suzan-Monti, M., Loze, B., Fonsart, J., … Delfraissy, J.-F. (2015). On-demand preexposure prophylaxis in men at high risk for HIV-1 infection. The New England Journal of Medicine, 373(23), 2237–2246. https://doi.org/10.1056/NEJMoa1506273
Antoni, Tremblay, C., Delaugerre, C., Charreau, I., Cua, E., Rojas Castro, D., Raffi, F., Chas, J., Huleux, T., Spire, B., Capitant, C., Cotte, L., Meyer, L., Molina, J.-M., Aboulker, J.-P., Bajos, N., Baril, J.-G., Besnier, M., Binesse, J., … Chidiac, C. (2020). On-demand pre-exposure prophylaxis with tenofovir disoproxil fumarate plus emtricitabine among men who have sex with men with less frequent sexual intercourse: a post-hoc analysis of the ANRS IPERGAY trial. The Lancet HIV, 7(2), e113–e120. https://doi.org/10.1016/S2352-3018(19)30341-8
Eisinger, Dieffenbach, C. W., & Fauci, A. S. (2019). HIV viral load and transmissibility of HIV infection: undetectable equals untransmittable. JAMA: The Journal of the American Medical Association, 321(5), 451–452. https://doi.org/10.1001/jama.2018.21167
Todd, Grosskurth, H., Changalucha, J., Obasi, A., Mosha, F., Balira, R., Orroth, K., Hugonnet, S., Pujades, M., Ross, D., Gavyole, A., Mabey, D., & Hayes, R. (2006). Risk factors influencing HIV infection incidence in a rural African population: a nested case-control study. The Journal of Infectious Diseases, 193(3), 458–466. https://doi.org/10.1086/499313
Wald, & Link, K. (2002). Risk of human immunodeficiency virus infection in herpes simplex virus type 2–seropositive persons: a meta-analysis. The Journal of Infectious Diseases, 185(1), 45–52. https://doi.org/10.1086/338231
Freeman, Weiss, H. A., Glynn, J. R., Cross, P. L., Whitworth, J. A., & Hayes, R. J. (2006). Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta-analysis of longitudinal studies. AIDS (London), 20(1), 73–83. https://doi.org/10.1097/01.aids.0000198081.09337.a7
Atashili, Poole, C., Ndumbe, P. M., Adimora, A. A., & Smith, J. S. (2008). Bacterial vaginosis and HIV acquisition: a meta-analysis of published studies. AIDS (London), 22(12), 1493–1501. https://doi.org/10.1097/QAD.0b013e3283021a37
Kissinger, & Adamski, A. (2013). Trichomoniasis and HIV interactions: a review. Sexually Transmitted Infections, 89(6), 426–433. https://doi.org/10.1136/sextrans-2012-051005
Centers for Disease Control and Prevention. (2018). Updated guidelines for antiretroviral postexposure prophylaxis after sexual, injection drug use, or other nonoccupational exposure to HIV—United States, 2016. U.S. Department of Health and Human Services. https://www.cdc.gov/hiv/pdf/programresources/cdc-hiv-npep-guidelines.pdf
Practice Bulletin No. 152: Emergency contraception. (2015). Obstetrics and Gynecology (New York. 1953), 126(3), e1–e11. https://doi.org/10.1097/AOG.0000000000001047
Mugo, Heffron, R., Donnell, D., Wald, A., Were, E. O., Rees, H., Celum, C., Kiarie, J. N., Cohen, C. R., Kayintekore, K., & Baeten, J. M. (2011). Increased risk of HIV-1 transmission in pregnancy: a prospective study among African HIV-1-serodiscordant couples. AIDS (London), 25(15), 1887–1895. https://doi.org/10.1097/QAD.0b013e32834a9338
Thomson, Hughes, J., Baeten, J. M., John-Stewart, G., Celum, C., Cohen, C. R., Ngure, K., Kiarie, J., Mugo, N., & Heffron, R. (2018). Increased risk of HIV acquisition among women throughout pregnancy and during the postpartum period: a prospective per-coital-act analysis among women with HIV-infected partners. The Journal of Infectious Diseases, 218(1), 16–25. https://doi.org/10.1093/infdis/jiy113
Mugwanya, John-Stewart, G., & Baeten, J. (2017). Safety of oral tenofovir disoproxil fumarate-based HIV pre-exposure prophylaxis use in lactating HIV-uninfected women. Expert Opinion on Drug Safety, 16(7), 867–871. https://doi.org/10.1080/14740338.2017.1338271
Benaboud, Pruvost, A., Coffie, P. A., Ekouévi, D. K., Urien, S., Arrivé, E., Blanche, S., Théodoro, F., Avit, D., Dabis, F., Tréluyer, J.-M., & Hirt, D. (2011). Concentrations of tenofovir and emtricitabine in breast milk of HIV-1-infected women in Abidjan, Côte d’Ivoire, in the ANRS 12109 TEmAA study, step 2. Antimicrobial Agents and Chemotherapy, 55(3), 1315–1317. https://doi.org/10.1128/AAC.00514-10
Waitt, Olagunju, A., Nakalema, S., Kyohaire, I., Owen, A., Lamorde, M., & Khoo, S. (20 18). Plasma and breast milk pharmacokinetics of emtricitabine, tenofovir and lamivudine using dried blood and breast milk spots in nursing African mother-infant pairs. Journal of Antimicrobial Chemotherapy, 73(4), 1013–1019. https://doi.org/10.1093/jac/dkx507
Jones, Restrepo, D., Kasowitz, A., Korenstein, D., Wallenstein, S., Schneider, A., & Keller, M. J. (2007). Risk factors for decreased bone density and effects of HIV on bone in the elderly. Osteoporosis International, 19(7), 913–918. https://doi.org/10.1007/s00198-007-0524-8
Cazanave, Dupon, M., Lavignolle-Aurillac, V., Barthe, N., Lawson-Ayayi, S., Mehsen, N., Mercié, P., Morlat, P., Thiébaut, R., & Dabis, F. (2008). Reduced bone mineral density in HIV-infected patients: prevalence and associated factors. AIDS (London), 22(3), 395–402. https://doi.org/10.1097/QAD.0b013e3282f423dd
Brown, & Qaqish, R. B. (2006). Antiretroviral therapy and the prevalence of osteopenia and osteoporosis: a meta-analytic review. AIDS (London), 20(17), 2165–2174. https://doi.org/10.1097/QAD.0b013e32801022eb
Solomon M. (2015). The safety of HIV pre-exposure prophylaxis in the presence of hepatitis B infection [Conference presentation abstract TUAC0201, 2015]. 8th International AIDS Society Conference on HIV Pathogenesis, Treatment, and Prevention (IAS 2015), Vancouver, Canada.
Pinkerton, & Abramson, P.. (1997). Effectiveness of condoms in preventing HIV transmission. AIDS Weekly (p. 37–). NewsRX LLC.
Sivay, Li, M., Piwowar-Manning, E., Zhang, Y., Hudelson, S. E., Marzinke, M. A., Amico, R. K., Redd, A., Hendrix, C. W., Anderson, P. L., Bokoch, K., Gail-Bekker, L., van Griensven, F., Mannheimer, S., Hughes, J. P., Grant, R., & Eshleman, S. H. (2017). Characterization of HIV seroconverters in a TDF/FTC PrEP study: HPTN 067/ADAPT. JAIDS-Journal of Acquired Immune Deficiency Syndromes, 75(3), 271–279. https://doi.org/10.1097/QAI.0000000000001374
Schillie, Wester, C., Osborne, M., Wesolowski, L., & Ryerson, A. B. (2020). CDC recommendations for hepatitis C screening among adults - United States, 2020. MMWR. Recommendations and Reports, 69(2), 1–17. https://doi.org/10.15585/mmwr.rr6902a1
Abara, Qaseem, A., Schillie, S., McMahon, B. J., & Harris, A. M. (2017). Hepatitis B vaccination, screening, and linkage to care: best practice advice from the American College of Physicians and the Centers for Disease Control and Prevention. Annals of Internal Medicine, 167(11), 794–804. https://doi.org/10.7326/M17-1106
Akkaya, Kiyici, M., Yilmaz, Y., Ulukaya, E., & Yerci, O. (2007). Clinical significance of activity of ALT enzyme in patients with hepatitis C virus. World Journal of Gastroenterology: WJG, 13(41), 5481–5485. https://doi.org/10.3748/wjg.v13.i41.5481
Finer, & Zolna, M. R. (2016). Declines in unintended pregnancy in the United States, 2008–2011. The New England Journal of Medicine, 374(9), 843–852. https://doi.org/10.1056/NEJMsa1506575
Ogbuagu, Ruane, P. J., Podzamczer, D., Salazar, L. C., Asmuth, D. M., Wohl, D., Gilson, R., Shao, Y., Cox, S., Kintu, A., Carter, C., Baeten, J. M., Brainard, D. M., Whitlock, G., Brunetta, J. M., Spinner, C. D., Antinori, A., Apea, V., Avery, A., … Brar, I. (2021). Long-term safety and efficacy of emtricitabine and tenofovir alafenamide vs emtricitabine and tenofovir disoproxil fumarate for HIV-1 pre-exposure prophylaxis: week 96 results from a randomised, double-blind, placebo-controlled, phase 3 trial. The Lancet HIV, 8(7), e397–e407. https://doi.org/10.1016/S2352-3018(21)00071-0
Nelson, Weng, M. K., Hofmeister, M. G., Moore, K. L., Doshani, M., Kamili, S., Koneru, A., Haber, P., Hagan, L., Romero, J. R., Schillie, S., & Harris, A. M. (2020). Prevention of hepatitis A virus infection in the United States: recommendations of the advisory committee on immunization practices, 2020. MMWR. Morbidity and Mortality Weekly Report. Recommendations and Reports, 69(5), 1–38. https://doi.org/10.15585/mmwr.rr6905a1
Schillie, Vellozzi, C., Reingold, A., Harris, A., Haber, P., Ward, J. W., & Nelson, N. P. (2018). Prevention of hepatitis B virus infection in the United States: recommendations of the advisory committee on immunization practices. MMWR. Recommendations and Reports, 67(1), 1–31. https://doi.org/10.15585/mmwr.rr6701a1
Knox, Tabrizi, S. N., Miller, P., Petoumenos, K., Law, M., Chen, S., & Garland, S. M. (2002). Evaluation of self-collected samples in contrast to practitioner-collected samples for detection of chlamydia trachomatis, neisseria gonorrhoeae, and trichomonas vaginalis by polymerase chain reaction among women living in remote areas. Sexually Transmitted Diseases, 29(11), 647–654. https://doi.org/10.1097/00007435-200211000-00006
Lunny, Taylor, D., Hoang, L., Wong, T., Gilbert, M., Lester, R., Krajden, M., Ogilvie, G., & Greub, G. (2015). Self-collected versus clinician-collected sampling for chlamydia and gonorrhea screening: a systemic review and meta-analysis. PloS One, 10(7), e0132776–e0132776. https://doi.org/10.1371/journal.pone.0132776
Dukers-Muijrers, Schachter, J., van Liere, G. A. F. ., Wolffs, P. F. ., & Hoebe, C. J. P. . (2015). What is needed to guide testing for anorectal and pharyngeal chlamydia trachomatis and neisseria gonorrhoeae in women and men? Evidence and opinion. BMC Infectious Diseases, 15(1), 533–533. https://doi.org/10.1186/s12879-015-1280-6
Gratrix, Singh, A. E., Bergman, J., Egan, C., Plitt, S. S., McGinnis, J., Bell, C. A., Drews, S. J., & Read, R. (2015). Evidence for increased chlamydia case finding after the introduction of rectal screening among women attending 2 Canadian sexually transmitted infection clinics. Clinical Infectious Diseases, 60(3), 398–404. https://doi.org/10.1093/cid/ciu831
Tittle, Boffito, M., McOwan, A., & Whitlock, G. (2020). Antiretroviral resistance and management after pre-exposure prophylaxis. The Lancet HIV, 7(2), e84–e84. https://doi.org/10.1016/S2352-3018(19)30404-7
Liegler, Abdel-Mohsen, M., Bentley, L. G., Atchison, R., Schmidt, T., Javier, J., Mehrotra, M., Eden, C., Glidden, D. V., McMahan, V., Anderson, P. L., Li, P., Wong, J. K., Buchbinder, S., Guanira, J. V., Grant, R. M., & iPrEx Study Team, S. T. (2014). HIV-1 drug resistance in the iPrEx preexposure prophylaxis trial. The Journal of Infectious Diseases, 210(8), 1217–1227. https://doi.org/10.1093/infdis/jiu233
Parikh, & Mellors, J. W. (2016). Should we fear resistance from tenofovir/emtricitabine preexposure prophylaxis? Current Opinion in HIV & AIDS, 11(1), 49–55. https://doi.org/10.1097/COH.0000000000000209
Abbas, Glaubius, R., Mubayi, A., Hood, G., & Mellors, J. W. (2013). Antiretroviral therapy and pre-exposure prophylaxis: combined impact on HIV transmission and drug resistance in South Africa. The Journal of Infectious Diseases, 208(2), 224–234. https://doi.org/10.1093/infdis/jit150
Baggaley, Powers, K. A., & Boily, M.-C. (2011). What do mathematical models tell us about the emergence and spread of drug-resistant HIV? Current Opinion in HIV & AIDS, 6(2), 131–140. https://doi.org/10.1097/COH.0b013e328343ad03
Gibas, van den Berg, P., Powell, V. E., & Krakower, D. S. (2019). Drug resistance during HIV pre-exposure prophylaxis. Drugs (New York, N.Y.), 79(6), 609–619. https://doi.org/10.1007/s40265-019-01108-x
U.S. Preventive Services Task Force. A & B Recommendations. https://www.uspreventiveservicestaskforce.org/uspstf/recommendation-topics/uspstf-a-and-b-recommendations
Marzinke, Grinsztejn, B., Fogel, J. M., Piwowar-Manning, E., Li, M., Weng, L., McCauley, M., Cummings, V., Ahmed, S., Haines, C. D., Bushman, L. R., Petropoulos, C., Persaud, D., Adeyeye, A., Kofron, R., Rinehart, A., St Clair, M., Rooney, J. F., Pryluka, D., … Eshleman, S. H. (2021). Characterization of Human Immunodeficiency Virus (HIV) Infection in Cisgender Men and Transgender Women Who Have Sex With Men Receiving Injectable Cabotegravir for HIV Prevention: HPTN 083. The Journal of Infectious Diseases, 224(9), 1581–1592. https://doi.org/10.1093/infdis/jiab152
Finer, & Zolna, M. R. (2016). Declines in Unintended Pregnancy in the United States, 2008–2011. The New England Journal of Medicine, 374(9), 843–852. https://doi.org/10.1056/NEJMsa1506575
Centers for Disease Control and Prevention. (n.d.). A guide to taking a sexual History. U.S. Department of Health and Human Services. https://www.cdc.gov/std/treatment/SexualHistory.pdf
National Coalition for Sexual Health. (2022). Sexual health and your patients: a provider’s guide. Altarum Institute. https://nationalcoalitionforsexualhealth.org/tools/for-healthcare-providers/asset/Provider-Guide_May-2022.pdf
AIDSVu. (n.d.). Deeper look: PrEP. https://aidsvu.org/resources/deeper-look-prep/
Centers for Disease Control and Prevention. (2018, March 6). 2018 CROI Prep Press release. Centers for Disease Control and Prevention. Retrieved September 13, 2022, from https://www.cdc.gov/nchhstp/newsroom/2018/croi-2018-PrEP-press-release.html
The BIPOC project. The BIPOC Project. (n.d.). Retrieved September 13, 2022, from https://www.thebipocproject.org/
Raypole, C. (2021, November 9). The difference between 'BIPOC' and 'POC' matters - here's why. Healthline. Retrieved September 15, 2022, from https://www.healthline.com/health/bipoc-meaning#what-it-stands-for
Centers for Disease Control and Prevention. (2022, August 26). HIV surveillance. Centers for Disease Control and Prevention. Retrieved September 15, 2022, from https://www.cdc.gov/hiv/library/reports/hiv-surveillance.html
Ryan J. Watson, Cindy J. Chang, Brian A. Feinstein, Raymond L. Moody, Antonia Caba & Lisa A. Eaton (2022) PrEP Stigma and logistical barriers remain significant challenges in curtailing HIV transmission among Black and Hispanic/Latinx cisgender sexual minority men and transgender women in the US, AIDS Care, DOI: 10.1080/09540121.2022.2098908
Watson, R. J., Chang, C. J., Feinstein, B. A., Moody, R. L., Caba, A., & Eaton, L. A. (2022). Prep stigma and logistical barriers remain significant challenges in curtailing HIV transmission among black and Hispanic/latinx cisgender sexual minority men and transgender women in the US. AIDS Care, 1–8. https://doi.org/10.1080/09540121.2022.2098908
Ogunbajo, A., Storholm, E. D., Ober, A. J., Bogart, L. M., Reback, C. J., Flynn, R., Lyman, P., & Morris, S. (2021). Multilevel barriers to HIV prep uptake and adherence among black and Hispanic/latinx transgender women in Southern California. AIDS and Behavior, 25(7), 2301–2315. https://doi.org/10.1007/s10461-021-03159-2
Ogunbajo, A., Storholm, E. D., Ober, A. J., Bogart, L. M., Reback, C. J., Flynn, R., Lyman, P., & Morris, S. (2021). Multilevel barriers to HIV prep uptake and adherence among black and Hispanic/latinx transgender women in Southern California. AIDS and Behavior, 25(7), 2301–2315. https://doi.org/10.1007/s10461-021-03159-2
Watson, R. J., Chang, C. J., Feinstein, B. A., Moody, R. L., Caba, A., & Eaton, L. A. (2022). Prep stigma and logistical barriers remain significant challenges in curtailing HIV transmission among black and Hispanic/latinx cisgender sexual minority men and transgender women in the US. AIDS Care, 1–8. https://doi.org/10.1080/09540121.2022.2098908
Bogart, L. M., Takada, S., & Cunningham, W. E. (2020). Medical Mistrust, discrimination, and the domestic HIV epidemic. HIV in US Communities of Color, 207–231. https://doi.org/10.1007/978-3-030-48744-7_12
Centers for Disease Control and Prevention. (2021, November 23). PrEP for HIV prevention in the U.S. Centers for Disease Control and Prevention. Retrieved September 15, 2022, from https://www.cdc.gov/nchhstp/newsroom/fact-sheets/hiv/PrEP-for-hiv-prevention-in-the-US-factsheet.html
Centers for Disease Control and Prevention. (2022, June 28). HIV and African American people. Centers for Disease Control and Prevention. Retrieved September 15, 2022, from https://www.cdc.gov/hiv/group/racialethnic/africanamericans/index.html
Centers for Disease Control and Prevention. (2022, August 18). HIV diagnoses. Centers for Disease Control and Prevention. Retrieved September 15, 2022, from https://www.cdc.gov/hiv/group/gender/women/diagnoses.html
Auerbach, J. D., Kinsky, S., Brown, G., & Charles, V. (2015). Knowledge, attitudes, and likelihood of pre-exposure prophylaxis (prep) use among US women at risk of acquiring HIV. AIDS Patient Care and STDs, 29(2), 102–110. https://doi.org/10.1089/apc.2014.0142
Ogunbajo, A., Storholm, E. D., Ober, A. J., Bogart, L. M., Reback, C. J., Flynn, R., Lyman, P., & Morris, S. (2021). Multilevel barriers to HIV prep uptake and adherence among black and Hispanic/latinx transgender women in Southern California. AIDS and Behavior, 25(7), 2301–2315. https://doi.org/10.1007/s10461-021-03159-2
Becasen, J. S., Denard, C. L., Mullins, M. M., Higa, D. H., & Sipe, T. A. (2019). Estimating the prevalence of HIV and sexual behaviors among the US transgender population: A systematic review and meta-analysis, 2006–2017. American Journal of Public Health, 109(1). https://doi.org/10.2105/ajph.2018.304727
Restar, A. J., Kuhns, L., Reisner, S. L., Ogunbajo, A., Garofalo, R., & Mimiaga, M. J. (2018). Acceptability of antiretroviral pre-exposure prophylaxis from a cohort of sexually experienced young transgender women in two U.S. cities. AIDS and Behavior, 22(11), 3649–3657. https://doi.org/10.1007/s10461-018-2127-0
Sevelius, J. M., Keatley, J. A., Calma, N., & Arnold, E. (2016). ‘I am not a man’: Trans-specific barriers and facilitators to prep acceptability among transgender women. Global Public Health, 11(7-8), 1060–1075. https://doi.org/10.1080/17441692.2016.1154085
Ogunbajo, A., Storholm, E. D., Ober, A. J., Bogart, L. M., Reback, C. J., Flynn, R., Lyman, P., & Morris, S. (2021). Multilevel barriers to HIV prep uptake and adherence among black and Hispanic/latinx transgender women in Southern California. AIDS and Behavior, 25(7), 2301–2315. https://doi.org/10.1007/s10461-021-03159-2
Eaton, L. A., Driffin, D. D., Bauermeister, J., Smith, H., & Conway-Washington, C. (2015). Minimal awareness and stalled uptake of pre-exposure prophylaxis (prep) among at risk, HIV-negative, black men who have sex with men. AIDS Patient Care and STDs, 29(8), 423–429. https://doi.org/10.1089/apc.2014.0303
Brooks, R. A., Landrian, A., Nieto, O., & Fehrenbacher, A. (2019). Experiences of anticipated and enacted pre-exposure prophylaxis (prep) stigma among Latino MSM in Los Angeles. AIDS and Behavior, 23(7), 1964–1973. https://doi.org/10.1007/s10461-019-02397-9
Centers for Disease Control and Prevention. (2022, June 28). HIV by age. Centers for Disease Control and Prevention. Retrieved September 15, 2022, from https://www.cdc.gov/hiv/group/age/index.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fhiv%2Fgroup%2Fage%2Fyouth%2Findex.html
Taggart, T., Liang, Y., Pina, P., & Albritton, T. (2020). Awareness of and willingness to use prep among black and Latinx adolescents residing in higher prevalence areas in the United States. PLOS ONE, 15(7). https://doi.org/10.1371/journal.pone.0234821
Centers for Disease Control and Prevention. (2022, August 26). HIV surveillance. Centers for Disease Control and Prevention. Retrieved September 16, 2022, from https://www.cdc.gov/hiv/library/reports/hiv-surveillance.html
National Indian Health Board. (n.d.). Retrieved September 16, 2022, from https://www.nihb.org/
Centers for Disease Control and Prevention. (2022, June 28). HIV in the United States by Race/Ethnicity. Centers for Disease Control and Prevention. Retrieved September 16, 2022, from https://www.cdc.gov/hiv/group/racialethnic/other-races/index.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fhiv%2Fgroup%2Fracialethnic%2Fasians%2Findex.html
Ending Asian & Pacific Islander HIV Stigma Starts here. Legacy Community Health. (2021, May 13). Retrieved September 16, 2022, from https://www.legacycommunityhealth.org/newsblog-ending-api-hiv-stigma-starts-here/
Wilberg, M. (2021, June 15). People who use drugs who are homeless good candidates for prep with appropriate support. aidsmap.com. Retrieved September 16, 2022, from https://www.aidsmap.com/news/jun-2021/people-who-use-drugs-who-are-homeless-good-candidates-prep-appropriate-support
Welcome to the California Overdose Surveillance Dashboard. CA Overdose Dashboard. (n.d.). Retrieved September 16, 2022, from https://skylab.cdph.ca.gov/ODdash/?tab=Home
CA Department of Public Health. (n.d.). Ending the HIV Epidemic. Ending the HIV Epidemic - CDC . Retrieved September 16, 2022, from https://www.cdph.ca.gov/Programs/CID/Pages/CID.aspx
Geter, A., Herron, A. R., & Sutton, M. Y. (2018). HIV-related stigma by healthcare providers in the United States: A systematic review. AIDS Patient Care and STDs, 32(10), 418–424. https://doi.org/10.1089/apc.2018.0114
Bunting, S. R., Feinstein, B. A., Calabrese, S. K., Hazra, A., Sheth, N. K., Chen, A. F., & Garber, S. S. (2022). Assumptions about patients seeking prep: Exploring the effects of patient and sexual partner race and Gender Identity and the moderating role of implicit racism. PLOS ONE, 17(7). https://doi.org/10.1371/journal.pone.0270861
Bogart, L. M., Takada, S., & Cunningham, W. E. (1970, January 1). Medical Mistrust, discrimination, and the domestic HIV epidemic. SpringerLink. Retrieved September 16, 2022, from https://link.springer.com/chapter/10.1007/978-3-030-48744-7_12
Martha Hostetter and Sarah Klein, “Understanding and Ameliorating Medical Mistrust Among Black Americans, “Transforming Care (newsletter), Commonwealth Fund, Jan 14, 2021. https://dou.org/10.26099/9grt-2b21
Alexander Green, M. D. (n.d.). 3 simple ways to alleviate patient mistrust. Quality Interactions. Retrieved January 10, 2022, from https://www.qualityinteractions.com/blog/3-simple-ways-to-alleviate-patient-mistrust
Nelson, A. (2002, August). Unequal treatment: Confronting racial and ethnic disparities in health care. Journal of the National Medical Association. Retrieved September 16, 2022, from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2594273/
Ojikutu, B. O., Amutah-Onukagha, N., Mahoney, T. F., Tibbitt, C., Dale, S. D., Mayer, K. H., & Bogart, L. M. (2020). HIV-related mistrust (or HIV conspiracy theories) and willingness to use prep among black women in the United States. AIDS and Behavior, 24(10), 2927–2934. https://doi.org/10.1007/s10461-020-02843-z
Bogart, L. M., Takada, S., & Cunningham, W. E. (2020). Medical Mistrust, discrimination, and the domestic HIV epidemic. HIV in US Communities of Color, 207–231. https://doi.org/10.1007/978-3-030-48744-7_12
AlRuthia, Y., Sales, I., Almalag, H., Alwhaibi, M., Almosabhi, L., Albassam, A. A., Alharbi, F. A., Bashatah, A., & Asiri, Y. (2020). the relationship between health-related quality of life and trust in primary care physicians among patients with diabetes. Clinical Epidemiology, Volume 12, 143–151. https://doi.org/10.2147/clep.s236952
Alpers, L.-M. (2016). Distrust and patients in Intercultural Healthcare: A qualitative interview study. Nursing Ethics, 25(3), 313–323. https://doi.org/10.1177/0969733016652449
Greer, T. M., Brondolo, E., & Brown, P. (2014). Systemic racism moderates effects of provider racial biases on adherence to hypertension treatment for African Americans. Health Psychology, 33(1), 35–42. https://doi.org/10.1037/a0032777
Bazargan, M., Cobb, S., & Assari, S. (2021). Discrimination and medical mistrust in a racially and ethnically diverse sample of California adults. The Annals of Family Medicine, 19(1), 4–15. https://doi.org/10.1370/afm.2632
Hilton, E. J., Lunardi, N., Sreedharan, R., Goff, K. L., Batakji, M., & Rosenberger, D. S. (2020). Two sides of the same coin. Anesthesiology Clinics, 38(2), 369–377. https://doi.org/10.1016/j.anclin.2020.01.001
Figure 18. percentage of all active physicians by Race/ethnicity, 2018. AAMC. (n.d.). Retrieved September 16, 2022, from https://www.aamc.org/data-reports/workforce/interactive-data/figure-18-percentage-all-active-physicians-race/ethnicity-2018
U.S. Census Bureau quickfacts: United States. (n.d.). Retrieved September 16, 2022, from https://www.census.gov/quickfacts/fact/table/US/PST045221
Batalova, J. B. J. (2021, May 14). Immigrant health-care workers in the United States. migrationpolicy.org. Retrieved September 16, 2022, from https://www.migrationpolicy.org/article/immigrant-health-care-workers-united-states-2018
Greenwood, H. (2021, November 16). Cultural diversity in Healthcare: USAHS. University of St. Augustine for Health Sciences. Retrieved September 16, 2022, from https://www.usa.edu/blog/diversity-in-healthcare/
Togioka, B. M., Duvivier, D., & Young, E. (2022). Diversity and Discrimination In Healthcare. In StatPearls. StatPearls Publishing.
Social Determinants of Health. Social Determinants of Health - Healthy People 2030. (n.d.). Retrieved September 16, 2022, from https://health.gov/healthypeople/priority-areas/social-determinants-health
Andriano, T. M., Arnsten, J., & Patel, V. V. (2022). Social Determinants of health and HIV pre-exposure prophylaxis (prep) interest and use among young black and Latinx Sexual Minority men. PLOS ONE, 17(4). https://doi.org/10.1371/journal.pone.0267031
Ogunbajo, A., Storholm, E. D., Ober, A. J., Bogart, L. M., Reback, C. J., Flynn, R., Lyman, P., & Morris, S. (2021, January 29). Multilevel barriers to HIV prep uptake and adherence among black and Hispanic/latinx transgender women in Southern California - AIDS and behavior. SpringerLink. Retrieved September 16, 2022, from https://link.springer.com/article/10.1007/s10461-021-03159-2
Understanding and addressing the social determinants of health for ... (n.d.). Retrieved September 16, 2022, from https://www.lgbtqiahealtheducation.org/wp-content/uploads/2019/06/TFIE-33_SDOHForBlackLGBTPeople_Web.pdf
10 strategies for creating LGBTQIA+ inclusive healthcare - cloudbreak. Martti by UpHealth. (2021, June 7). Retrieved September 16, 2022, from https://www.martti.us/2021/06/07/ten-strategies-for-creating-inclusive-healthcare-environments-for-lgbtqia-people/
Salabarría-Peña, Y., Douglas, C., Brantley, M., & Johnson, A. K. (2021, September 2). Informing the future of prep navigation: Findings from a five-site cluster evaluation. Evaluation and Program Planning. Retrieved September 16, 2022, from https://www.sciencedirect.com/science/article/pii/S014971892100094X